36H: A Novel Potent Inhibitor for Antimelanogenesis
- PMID: 29507652
- PMCID: PMC5817369
- DOI: 10.1155/2018/6354972
36H: A Novel Potent Inhibitor for Antimelanogenesis
Abstract
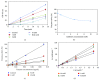
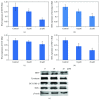
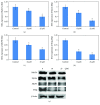
N-Hydroxycinnamoylphenalkylamides (36H) exhibited both antioxidation and antityrosinase abilities. The compound was studied for its antioxidative properties, using a 1,1-diphenyl-2-picrylhydrazul- (DPPH-) scavenging test, a ferric ion-reducing antioxidant power assay (FRAP) assessment, and a metal-chelating power assay. The results showed that 36H had antioxidative capabilities in the DPPH-scavenging and ferric-reducing power examinations but the chelating power assay did not demonstrate antioxidative capability. 36H was also measured for tyrosinase inhibitory activity applying various species platforms, including in vitro mushroom, B16F10 mouse melanoma, and human melanocyte cells. In terms of in vitro mushroom tyrosinase suppression, 36H restrained the melanogenesis processes. It is assumed that 36H blocked the tyrosinase active site as a competitive inhibitor for mushroom tyrosinase, hence not decreasing the human normal melanocyte cellular viability. A quantitative real-time polymerase chain reaction (qRT-PCR) and western blot discovered that 36H downregulated melanogenesis-related RNA and proteins, including pigment production (MITF, tyrosinase, TRP-1, and TRP-2), melanosome maturation (Rab27a), and melanosome transportation (Myo5a, MLPH and Mreg). Overall, 36H displayed the biofunctions of antioxidation and melanin suppression, so there was a possibility for its application as a food additive or a skin-whitening agent.
Figures




Similar articles
-
Inhibitory effects of adlay extract on melanin production and cellular oxygen stress in B16F10 melanoma cells.Int J Mol Sci. 2014 Sep 19;15(9):16665-79. doi: 10.3390/ijms150916665. Int J Mol Sci. 2014. PMID: 25244016 Free PMC article.
-
Ethyl acetate extract from Panax ginseng C.A. Meyer and its main constituents inhibit α-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells.J Ethnopharmacol. 2017 Aug 17;208:149-156. doi: 10.1016/j.jep.2017.07.004. Epub 2017 Jul 8. J Ethnopharmacol. 2017. PMID: 28689798
-
Acanthoic acid inhibits melanogenesis through tyrosinase downregulation and melanogenic gene expression in B16 melanoma cells.Nat Prod Commun. 2013 Oct;8(10):1359-62. Nat Prod Commun. 2013. PMID: 24354173
-
The hunt for natural skin whitening agents.Int J Mol Sci. 2009 Dec 10;10(12):5326-5349. doi: 10.3390/ijms10125326. Int J Mol Sci. 2009. PMID: 20054473 Free PMC article. Review.
-
Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors.J Enzyme Inhib Med Chem. 2017 Dec;32(1):403-425. doi: 10.1080/14756366.2016.1256882. J Enzyme Inhib Med Chem. 2017. PMID: 28097901 Free PMC article. Review.
Cited by
-
Isokotomolide A from Cinnamomum kotoense Induce Melanoma Autophagy and Apoptosis In Vivo and In Vitro.Oxid Med Cell Longev. 2020 Sep 26;2020:3425147. doi: 10.1155/2020/3425147. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33062137 Free PMC article.
-
Purified Astaxanthin from Haematococcus pluvialis Promotes Tissue Regeneration by Reducing Oxidative Stress and the Secretion of Collagen In Vitro and In Vivo.Oxid Med Cell Longev. 2020 Aug 3;2020:4946902. doi: 10.1155/2020/4946902. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32832000 Free PMC article.
-
A Novel Oral Astaxanthin Nanoemulsion from Haematococcus pluvialis Induces Apoptosis in Lung Metastatic Melanoma.Oxid Med Cell Longev. 2020 Aug 26;2020:2647670. doi: 10.1155/2020/2647670. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32908627 Free PMC article.
-
Antioxidant Graphene Oxide Nanoribbon as a Novel Whitening Agent Inhibits Microphthalmia-Associated Transcription Factor-Related Melanogenesis Mechanism.ACS Omega. 2020 Mar 19;5(12):6588-6597. doi: 10.1021/acsomega.9b04316. eCollection 2020 Mar 31. ACS Omega. 2020. PMID: 32258894 Free PMC article.
-
Adipose-Derived Stem Cell-Incubated HA-Rich Sponge Matrix Implant Modulates Oxidative Stress to Enhance VEGF and TGF-β Secretions for Extracellular Matrix Reconstruction In Vivo.Oxid Med Cell Longev. 2022 Jan 17;2022:9355692. doi: 10.1155/2022/9355692. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35082971 Free PMC article.
References
-
- Wang H. M. Bioconstituents from stems of Synsepalum Dulcificum Daniell (Sapotaceae) inhibit human melanoma proliferation, reduce mushroom tyrosinase activity and have antioxidant properties. Journal of the Taiwan Institute of Chemical Engineers. 2011;42(2):204–211. doi: 10.1016/j.jtice.2010.05.008. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

