Nocturnal Hypoxia Improves Glucose Disposal, Decreases Mitochondrial Efficiency, and Increases Reactive Oxygen Species in the Muscle and Liver of C57BL/6J Mice Independent of Weight Change
- PMID: 29507654
- PMCID: PMC5817288
- DOI: 10.1155/2018/9649608
Nocturnal Hypoxia Improves Glucose Disposal, Decreases Mitochondrial Efficiency, and Increases Reactive Oxygen Species in the Muscle and Liver of C57BL/6J Mice Independent of Weight Change
Abstract
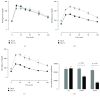
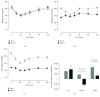
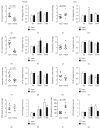
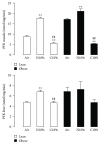
Although acute exposure to hypoxia can disrupt metabolism, longer-term exposure may normalize glucose homeostasis or even improve glucose disposal in the presence of obesity. We examined the effects of two-week exposure to room air (Air), continuous 10% oxygen (C10%), and 12 hr nocturnal periods of 10% oxygen (N10%) on glucose disposal, insulin responsiveness, and mitochondrial function in lean and obese C57BL/6J mice. Both C10% and N10% improved glucose disposal relative to Air in lean and obese mice without evidence of an increase in insulin responsiveness; however, only the metabolic improvements with N10% exposure occurred in the absence of confounding effects of weight loss. In lean mice, N10% exposure caused a decreased respiratory control ratio (RCR) and increased reactive oxygen species (ROS) production in the mitochondria of the muscle and liver compared to Air-exposed mice. In the absence of hypoxia, obese mice exhibited a decreased RCR in the muscle and increased ROS production in the liver compared to lean mice; however, any additional effects of hypoxia in the presence of obesity were minimal. Our data suggest that the development of mitochondrial inefficiency may contribute to metabolic adaptions to hypoxia, independent of weight, and metabolic adaptations to adiposity, independent of hypoxia.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

