Evaluation of SAS1B as a target for antibody-drug conjugate therapy in the treatment of pancreatic cancer
- PMID: 29507667
- PMCID: PMC5823626
- DOI: 10.18632/oncotarget.23944
Evaluation of SAS1B as a target for antibody-drug conjugate therapy in the treatment of pancreatic cancer
Abstract
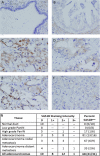
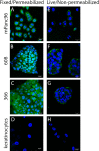
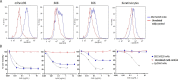
Successful therapeutic options remain elusive for pancreatic cancer. The exquisite sensitivity and specificity of humoral and cellular immunity may provide therapeutic approaches if antigens specific for pancreatic cancer cells can be identified. Here we characterize SAS1B (ovastacin, ASTL, astacin-like), a cancer-oocyte antigen, as an attractive immunotoxin target expressed at the surface of human pancreatic cancer cells, with limited expression among normal tissues. Immunohistochemistry shows that most pancreatic cancers are SAS1Bpos (68%), while normal pancreatic ductal epithelium is SAS1Bneg. Pancreatic cancer cell lines developed from patient-derived xenograft models display SAS1B cell surface localization, in addition to cytoplasmic expression, suggesting utility for SAS1B in multiple immunotherapeutic approaches. When pancreatic cancer cells were treated with an anti-SAS1B antibody-drug conjugate, significant cell death was observed at 0.01-0.1 μg/mL, while SAS1Bneg human keratinocytes were resistant. Cytotoxicity was correlated with SAS1B cell surface expression; substantial killing was observed for tumors with low steady state SAS1B expression, suggesting a substantial proportion of SAS1Bpos tumors can be targeted in this manner. These results demonstrate SAS1B is a surface target in pancreatic cancer cells capable of binding monoclonal antibodies, internalization, and delivering cytotoxic drug payloads, supporting further development of SAS1B as a novel target for pancreatic cancer.
Keywords: ASTL/SAS1B/ovastacin; antibody-drug conjugate; pancreatic cancer biomarker; surface cancer-oocyte antigen; targeted immunotherapy.
Conflict of interest statement
CONFLICTS OF INTEREST The University of Virginia has filed patent applications on the use of SAS1B as a cancer drug and diagnostic target with KAK, ESP, AM, and late JCH listed as inventors.
Figures

References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. https://doi.org/10.1158/0008-5472.CAN-14-0155. - DOI - PubMed
-
- Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182–98. https://doi.org/10.3748/wjg.v20.i32.11182. - DOI - PMC - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. https://doi.org/10.3322/caac.21208. - DOI - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. https://doi.org/10.3322/caac.21387. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

