Analysis of a gene panel for targeted sequencing of colorectal cancer samples
- PMID: 29507673
- PMCID: PMC5823670
- DOI: 10.18632/oncotarget.24138
Analysis of a gene panel for targeted sequencing of colorectal cancer samples
Abstract
Colorectal cancer (CRC) is a leading cause of death worldwide. Surgical intervention is a successful treatment for stage I patients, whereas other more advanced cases may require adjuvant chemotherapy. The selection of effective adjuvant treatments remains, however, challenging. Accurate patient stratification is necessary for the identification of the subset of patients likely responding to treatment, while sparing others from pernicious treatment. Targeted sequencing approaches may help in this regard, enabling rapid genetic investigation, and at the same time easily applicable in routine diagnosis. We propose a set of guidelines for the identification, including variant calling and filtering, of somatic mutations driving tumorigenesis in the absence of matched healthy tissue. We also discuss the inclusion criteria for the generation of our gene panel. Furthermore, we evaluate the prognostic impact of individual genes, using Cox regression models in the context of overall survival and disease-free survival. These analyses confirmed the role of commonly used biomarkers, and shed light on controversial genes such as CYP2C8. Applying those guidelines, we created a novel gene panel to investigate the onset and progression of CRC in 273 patients. Our comprehensive biomarker set includes 266 genes that may play a role in the progression through the different stages of the disease. Tracing the developmental state of the tumour, and its resistances, is instrumental in patient stratification and reliable decision making in precision clinical practice.
Keywords: NGS; biomarker discovery; colorectal cancer; precision medicine.
Conflict of interest statement
CONFLICTS OF INTEREST TSJ, RWE and ERH are part of the Intomics management group. TSJ and RWE are shareholders of Intomics. The rest of authors declare no conflicts of interest.
Figures
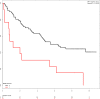

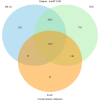
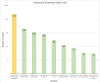
References
-
- De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12:594–603. - PubMed
-
- Modest DP, Brodowicz T, Stintzing S, Jung A, Neumann J, Laubender RP, Ocvirk J, Kurteva G, Papai Z, Knittelfelder R, Kirchner T, Heinemann V, Zielinski CC. Impact of the specific mutation in KRAS codon 12 mutated tumors on treatment efficacy in patients with metastatic colorectal cancer receiving cetuximab-based first-line therapy: a pooled analysis of three trials. Oncology. 2012;83:241–7. - PubMed
-
- Dienstmann R, Mason MJ, Sinicrope FA, Phipps AI, Tejpar S, Nesbakken A, Danielsen SA, Sveen A, Buchanan DD, Clendenning M, Rosty C, Bot B, Alberts SR, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28:1023–31. - PMC - PubMed
-
- Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C, Lindor NM, Minsky BD, Monzon FA, Sargent DJ, Singh VM, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453–86. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

