Population-based mechanistic modeling allows for quantitative predictions of drug responses across cell types
- PMID: 29507757
- PMCID: PMC5825396
- DOI: 10.1038/s41540-018-0047-2
Population-based mechanistic modeling allows for quantitative predictions of drug responses across cell types
Abstract
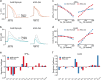
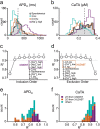
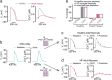
Quantitative mismatches between human physiology and experimental models can be problematic for the development of effective therapeutics. When the effects of drugs on human adult cardiac electrophysiology are of interest, phenotypic differences with animal cells, and more recently stem cell-derived models, can present serious limitations. We addressed this issue through a combination of mechanistic mathematical modeling and statistical analyses. Physiological metrics were simulated in heterogeneous populations of models describing cardiac myocytes from adult ventricles and those derived from induced pluripotent stem cells (iPSC-CMs). These simulated measures were used to construct a cross-cell type regression model that predicts adult myocyte drug responses from iPSC-CM behaviors. We found that (1) quantitatively accurate predictions of responses to selective or non-selective ion channel blocking drugs could be generated based on iPSC-CM responses under multiple experimental conditions; (2) altering extracellular ion concentrations is an effective experimental perturbation for improving the model's predictive strength; (3) the method can be extended to predict and contrast drug responses in diseased as well as healthy cells, indicating a broader application of the concept. This cross-cell type model can be of great value in drug development, and the approach, which can be applied to other fields, represents an important strategy for overcoming experimental model limitations.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Predicting individual-specific cardiotoxicity responses induced by tyrosine kinase inhibitors.Front Pharmacol. 2023 Apr 10;14:1158222. doi: 10.3389/fphar.2023.1158222. eCollection 2023. Front Pharmacol. 2023. PMID: 37101545 Free PMC article.
-
Tailoring Mathematical Models to Stem-Cell Derived Cardiomyocyte Lines Can Improve Predictions of Drug-Induced Changes to Their Electrophysiology.Front Physiol. 2017 Dec 12;8:986. doi: 10.3389/fphys.2017.00986. eCollection 2017. Front Physiol. 2017. PMID: 29311950 Free PMC article.
-
Differential responses of induced pluripotent stem cell-derived cardiomyocytes to anisotropic strain depends on disease status.J Biomech. 2015 Nov 5;48(14):3890-6. doi: 10.1016/j.jbiomech.2015.09.028. Epub 2015 Oct 8. J Biomech. 2015. PMID: 26476764 Free PMC article.
-
The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1.Pharmacol Ther. 2018 Mar;183:127-136. doi: 10.1016/j.pharmthera.2017.10.001. Epub 2017 Oct 3. Pharmacol Ther. 2018. PMID: 28986101 Review.
-
MicroRNA-mediated maturation of human pluripotent stem cell-derived cardiomyocytes: Towards a better model for cardiotoxicity?Food Chem Toxicol. 2016 Dec;98(Pt A):17-24. doi: 10.1016/j.fct.2016.05.025. Epub 2016 Jun 3. Food Chem Toxicol. 2016. PMID: 27265266 Review.
Cited by
-
Visualization of currents in neural models with similar behavior and different conductance densities.Elife. 2019 Jan 31;8:e42722. doi: 10.7554/eLife.42722. Elife. 2019. PMID: 30702427 Free PMC article.
-
Neuronal population models reveal specific linear conductance controllers sufficient to rescue preclinical disease phenotypes.iScience. 2021 Oct 14;24(11):103279. doi: 10.1016/j.isci.2021.103279. eCollection 2021 Nov 19. iScience. 2021. PMID: 34778727 Free PMC article.
-
Building blocks of microphysiological system to model physiology and pathophysiology of human heart.Front Physiol. 2023 Jul 6;14:1213959. doi: 10.3389/fphys.2023.1213959. eCollection 2023. Front Physiol. 2023. PMID: 37485060 Free PMC article. Review.
-
Quantitative analysis of variability in an integrated model of human ventricular electrophysiology and β-adrenergic signaling.J Mol Cell Cardiol. 2020 Jun;143:96-106. doi: 10.1016/j.yjmcc.2020.04.009. Epub 2020 Apr 21. J Mol Cell Cardiol. 2020. PMID: 32330487 Free PMC article.
-
All-Optical Electrophysiology Refines Populations of In Silico Human iPSC-CMs for Drug Evaluation.Biophys J. 2020 May 19;118(10):2596-2611. doi: 10.1016/j.bpj.2020.03.018. Epub 2020 Apr 4. Biophys J. 2020. PMID: 32298635 Free PMC article.
References
-
- van den Heuvel NH, van Veen TA, Lim B, Jonsson MK. Lessons from the heart: mirroring electrophysiological characteristics during cardiac development to in vitro differentiation of stem cell derived cardiomyocytes. J. Mol. Cell. Cardiol. 2014;67:12–25. doi: 10.1016/j.yjmcc.2013.12.011. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

