Human anti-HIV IgM detection by the OraQuick ADVANCE® Rapid HIV 1/2 Antibody Test
- PMID: 29507828
- PMCID: PMC5834934
- DOI: 10.7717/peerj.4430
Human anti-HIV IgM detection by the OraQuick ADVANCE® Rapid HIV 1/2 Antibody Test
Erratum in
-
Correction: Human anti-HIV IgM detection by the OraQuick ADVANCE® Rapid HIV 1/2 Antibody Test.PeerJ. 2018 Jun 22;6:e4430/correction-1. doi: 10.7717/peerj.4430/correction-1. eCollection 2018. PeerJ. 2018. PMID: 29942672 Free PMC article.
Abstract
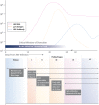
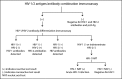
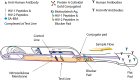
The Centers for Disease Control and Prevention (CDC) and many public health jurisdictions continue to advocate for the most sensitive rapid HIV test that is available. Currently, the recommendation is to utilize tests that can detect HIV infection biomarkers within 30 days of infection, when initial immune responses are mounted. The infected patient's IgM response is often used to detect acute infection within a 20-25 days window after infection. This requirement applies to lab-based testing with automated analyzers and rapid, point of care (POC) testing used for screening in a non-clinical setting. A recent study has demonstrated that POC tests using a Protein A-based detection system can detect samples with predominantly HIV-1 IgM reactivity (Moshgabadi et al., 2015). The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test (OraQuick ADVANCE®) also uses Protein A as the detection protein in the antibody-binding colloidal gold conjugate, so it is expected that the OraQuick ADVANCE® Test will also detect samples with predominantly IgM reactivity. This report definitively demonstrates that the OraQuick ADVANCE® Test can detect IgM antibodies during an acute infection window period of approximately 20-25 days after infection, and is therefore suitable for use in testing environments requiring adherence to current CDC recommendations.
Keywords: CDC; HIV; IgM detection; POC; Rapid test; Seroconversion.
Conflict of interest statement
Geraldine Guillon, Graham Yearwood, Casey Snipes, Daniel Boschi and Michael R. Reed are employees of OraSure Technologies, Inc.
Figures
References
-
- Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations 2014. https://stacks.cdc.gov/view/cdc/23447 https://stacks.cdc.gov/view/cdc/23447
-
- Centers for Disease Control and Prevention. Implementing HIV Testing in Nonclinical Settings: A Guide for HIV Testing Providers Center for Disease Control and Prevention. 2016. https://www.cdc.gov/hiv/pdf/testing/CDC_HIV_Implementing_HIV_Testing_in_... https://www.cdc.gov/hiv/pdf/testing/CDC_HIV_Implementing_HIV_Testing_in_...
-
- Conway DP, Holt M, McNulty A, Couldwell DL, Smith DE, Davies SC, Cunningham P, Keen P, Guy R, Sydney Rapid HIV Test Study Multi-centre evaluation of the determine HIV combo assay when used for point of care testing in a high risk clinic-based population. PLOS ONE. 2014;9(4):e94062. doi: 10.1371/journal.pone.0094062. - DOI - PMC - PubMed
-
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–1879. doi: 10.1097/00002030-200309050-00005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

