Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders?
- PMID: 29507865
- PMCID: PMC5821995
- DOI: 10.1155/2018/8917804
Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders?
Abstract
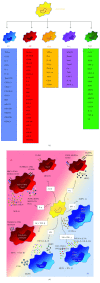
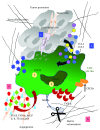
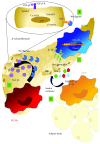
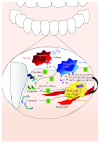
Macrophages are key cellular components of the innate immunity, acting as the main player in the first-line defence against the pathogens and modulating homeostatic and inflammatory responses. Plasticity is a major feature of macrophages resulting in extreme heterogeneity both in normal and in pathological conditions. Macrophages are not homogenous, and they are generally categorized into two broad but distinct subsets as either classically activated (M1) or alternatively activated (M2). However, macrophages represent a continuum of highly plastic effector cells, resembling a spectrum of diverse phenotype states. Induction of specific macrophage functions is closely related to the surrounding environment that acts as a relevant orchestrator of macrophage functions. This phenomenon, termed polarization, results from cell/cell, cell/molecule interaction, governing macrophage functionality within the hosting tissues. Here, we summarized relevant cellular and molecular mechanisms driving macrophage polarization in "distant" pathological conditions, such as cancer, type 2 diabetes, atherosclerosis, and periodontitis that share macrophage-driven inflammation as a key feature, playing their dual role as killers (M1-like) and/or builders (M2-like). We also dissect the physio/pathological consequences related to macrophage polarization within selected chronic inflammatory diseases, placing polarized macrophages as a relevant hallmark, putative biomarkers, and possible target for prevention/therapy.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

