Antibiotic Stimulation of a Bacillus subtilis Migratory Response
- PMID: 29507890
- PMCID: PMC5821984
- DOI: 10.1128/mSphere.00586-17
Antibiotic Stimulation of a Bacillus subtilis Migratory Response
Abstract
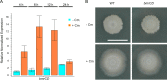
Competitive interactions between bacteria reveal physiological adaptations that benefit fitness. Bacillus subtilis is a Gram-positive species with several adaptive mechanisms for competition and environmental stress. Biofilm formation, sporulation, and motility are the outcomes of widespread changes in a population of B. subtilis. These changes emerge from complex, regulated pathways for adapting to external stresses, including competition from other species. To identify competition-specific functions, we cultured B. subtilis with multiple species of Streptomyces and observed altered patterns of growth for each organism. In particular, when plated on agar medium near Streptomyces venezuelae, B. subtilis initiates a robust and reproducible mobile response. To investigate the mechanistic basis for the interaction, we determined the type of motility used by B. subtilis and isolated inducing metabolites produced by S. venezuelae. Bacillus subtilis has three defined forms of motility: swimming, swarming, and sliding. Streptomyces venezuelae induced sliding motility specifically in our experiments. The inducing agents produced by S. venezuelae were identified as chloramphenicol and a brominated derivative at subinhibitory concentrations. Upon further characterization of the mobile response, our results demonstrated that subinhibitory concentrations of chloramphenicol, erythromycin, tetracycline, and spectinomycin all activate a sliding motility response by B. subtilis. Our data are consistent with sliding motility initiating under conditions of protein translation stress. This report underscores the importance of hormesis as an early warning system for potential bacterial competitors and antibiotic exposure. IMPORTANCE Antibiotic resistance is a major challenge for the effective treatment of infectious diseases. Identifying adaptive mechanisms that bacteria use to survive low levels of antibiotic stress is important for understanding pathways to antibiotic resistance. Furthermore, little is known about the effects of individual bacterial interactions on multispecies communities. This work demonstrates that subinhibitory amounts of some antibiotics produced by streptomycetes induce active motility in B. subtilis, which may alter species interaction dynamics among species-diverse bacterial communities in natural environments. The use of antibiotics at subinhibitory concentrations results in many changes in bacteria, including changes in biofilm formation, small-colony variants, formation of persisters, and motility. Identifying the mechanistic bases of these adaptations is crucial for understanding how bacterial communities are impacted by antibiotics.
Keywords: Bacillus subtilis; Streptomyces venezuelae; antibiotics; chloramphenicol; competition; hormesis; ribosome; sliding motility.
Figures






Similar articles
-
Linearmycins Activate a Two-Component Signaling System Involved in Bacterial Competition and Biofilm Morphology.J Bacteriol. 2017 Aug 22;199(18):e00186-17. doi: 10.1128/JB.00186-17. Print 2017 Sep 15. J Bacteriol. 2017. PMID: 28461449 Free PMC article.
-
Inhibition of Cell Differentiation in Bacillus subtilis by Pseudomonas protegens.J Bacteriol. 2015 Jul;197(13):2129-2138. doi: 10.1128/JB.02535-14. Epub 2015 Mar 30. J Bacteriol. 2015. PMID: 25825426 Free PMC article.
-
Escape from Lethal Bacterial Competition through Coupled Activation of Antibiotic Resistance and a Mobilized Subpopulation.PLoS Genet. 2015 Dec 8;11(12):e1005722. doi: 10.1371/journal.pgen.1005722. eCollection 2015 Dec. PLoS Genet. 2015. PMID: 26647299 Free PMC article.
-
Hormetic dose responses induced by antibiotics in bacteria: A phantom menace to be thoroughly evaluated to address the environmental risk and tackle the antibiotic resistance phenomenon.Sci Total Environ. 2021 Dec 1;798:149255. doi: 10.1016/j.scitotenv.2021.149255. Epub 2021 Jul 27. Sci Total Environ. 2021. PMID: 34340082 Review.
-
General and regulatory proteolysis in Bacillus subtilis.Subcell Biochem. 2013;66:73-103. doi: 10.1007/978-94-007-5940-4_4. Subcell Biochem. 2013. PMID: 23479438 Review.
Cited by
-
Bacillus Responses to Plant-Associated Fungal and Bacterial Communities.Front Microbiol. 2020 Jun 23;11:1350. doi: 10.3389/fmicb.2020.01350. eCollection 2020. Front Microbiol. 2020. PMID: 32655531 Free PMC article. Review.
-
Interspecies Social Spreading: Interaction between Two Sessile Soil Bacteria Leads to Emergence of Surface Motility.mSphere. 2019 Jan 30;4(1):e00696-18. doi: 10.1128/mSphere.00696-18. mSphere. 2019. PMID: 30700513 Free PMC article.
-
Surfactin facilitates establishment of Bacillus subtilis in synthetic communities.ISME J. 2025 Jan 2;19(1):wraf013. doi: 10.1093/ismejo/wraf013. ISME J. 2025. PMID: 39846898 Free PMC article.
-
Emerging evolutionary paradigms in antibiotic discovery.J Ind Microbiol Biotechnol. 2019 Mar;46(3-4):257-271. doi: 10.1007/s10295-018-2085-6. Epub 2018 Sep 29. J Ind Microbiol Biotechnol. 2019. PMID: 30269177 Review.
-
Exploration of Social Spreading Reveals That This Behavior Is Prevalent among Pedobacter and Pseudomonas fluorescens Isolates and That There Are Variations in the Induction of the Phenotype.Appl Environ Microbiol. 2021 Sep 10;87(19):e0134421. doi: 10.1128/AEM.01344-21. Epub 2021 Jul 21. Appl Environ Microbiol. 2021. PMID: 34288708 Free PMC article.
References
-
- Brunelle BW, Bearson BL, Bearson SMD. 2015. Chloramphenicol and tetracycline decrease motility and increase invasion and attachment gene expression in specific isolates of multidrug-resistant Salmonella enterica serovar Typhimurium. Front Microbiol 5:801. doi:10.3389/fmicb.2014.00801. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
