Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice
- PMID: 29507902
- PMCID: PMC5831023
- DOI: 10.1002/hep4.1151
Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice
Abstract
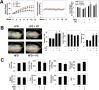
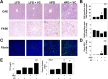
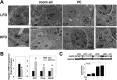
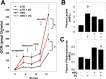
Vinyl chloride (VC), a common industrial organochlorine and environmental pollutant, has been shown to directly cause hepatic angiosarcoma and toxicant-associated steatohepatitis at high exposure levels. However, the impact of lower concentrations of VC on the progression of underlying liver diseases (e.g., nonalcoholic fatty liver disease [NAFLD]) is unclear. Given the high prevalence of NAFLD in the United States (and worldwide) population, this is an important concern. Recent studies by our group with VC metabolites suggest a potential interaction between VC exposure and underlying liver disease to cause enhanced damage. Here, a novel mouse model determined the effects of VC inhalation at levels below the current Occupational Safety and Health Administration limit (<1 ppm) in the context of NAFLD to better mimic human exposure and identify potential mechanisms of VC-induced liver injury. VC exposure caused no overt liver injury in mice fed a low-fat diet. However, in mice fed a high-fat diet (HFD), VC significantly increased liver damage, steatosis, and increased neutrophil infiltration. Moreover, VC further enhanced HFD-induced oxidative and endoplasmic reticulum stress. Importantly, VC exposure dysregulated energy homeostasis and impaired mitochondrial function, even in mice fed a low-fat diet. In toto, the results indicate that VC exposure causes metabolic stress that sensitizes the liver to steatohepatitis caused by HFD. Conclusion: The hypothesis that low-level (below the Occupational Safety and Health Administration limit) chronic exposure to VC by inhalation enhances liver injury caused by an HFD is supported. Importantly, our data raise concerns about the potential for overlap between fatty diets (i.e., Western diet) and exposure to VC and the health implications of this co-exposure for humans. It also emphasizes that current safety restrictions may be insufficient to account for other factors that can influence hepatotoxicity. (Hepatology Communications 2018;2:270-284).
Figures



References
-
- Agency for Toxic Substances and Disease Registry (ATSDR) . Toxicological profile for vinyl chloride. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2006.
-
- McKone TE, Knezovich JP. The transfer of trichloroethylene (TCE) from a shower to indoor air: experimental measurements and their implications. J Air Waste Manage Assoc 1991;41:282‐286. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

