I-124 codrituzumab imaging and biodistribution in patients with hepatocellular carcinoma
- PMID: 29508107
- PMCID: PMC5838028
- DOI: 10.1186/s13550-018-0374-8
I-124 codrituzumab imaging and biodistribution in patients with hepatocellular carcinoma
Abstract
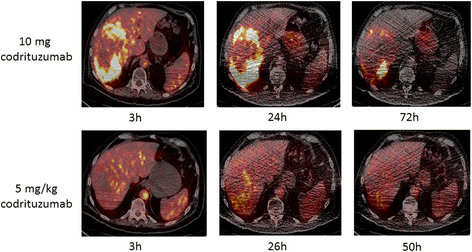
Background: I-124 codrituzumab (aka GC33), an antibody directed at Glypican 3, was evaluated in patients with hepatocellular carcinoma (HCC). Fourteen patients with HCC underwent baseline imaging with I-124 codrituzumab (~ 185 MBq, 10 mg). Seven of these patients undergoing sorafenib/immunotherapy with 2.5 or 5 mg/kg of cold codrituzumab had repeat imaging, with co-infusion of I-124 codrituzumab, as part of their immunotherapy treatment. Three patients who progressed while on sorafenib/immunotherapy were re-imaged after a 4-week washout period to assess for the presence of antigen. Serial positron emission tomography (PET) imaging and pharmacokinetics were performed following I-124 codrituzumab. An ELISA assay was used to determine "cold" codrituzumab serum pharmacokinetics and compare it to that of I-124 codrituzumab. Correlation of imaging results was performed with IHC. Short-term safety assessment was also evaluated.
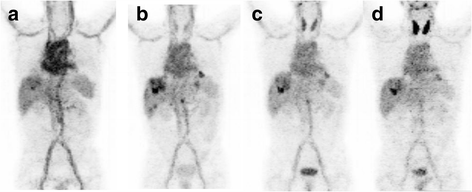
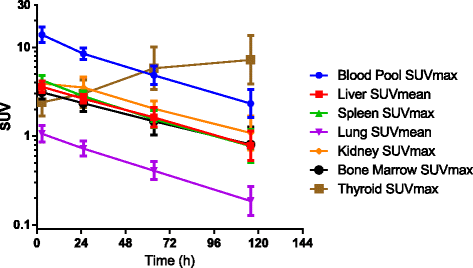
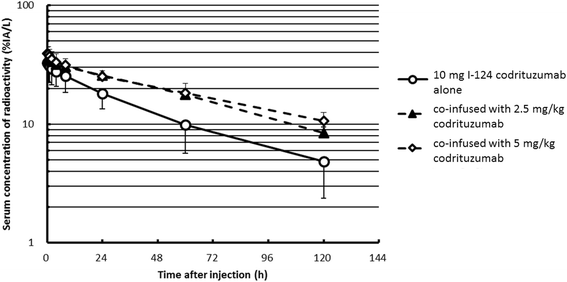
Results: Thirteen patients had tumor localization on baseline I-124 codrituzumab; heterogeneity in tumor uptake was noted. In three patients undergoing repeat imaging while on immunotherapy/sorafenib, evidence of decreased I-124 codrituzumab uptake was noted. All three patients who underwent imaging after progression while on immunotherapy continued to have I-124 codrituzumab tumor uptake. Pharmacokinetics of I-124 codrituzumab was similar to that of other intact IgG. No significant adverse events were observed related to the I-124 codrituzumab.
Conclusions: I-124 codrituzumab detected tumor localization in most patients with HCC. Pharmacokinetics was similar to that of other intact iodinated humanized IgG. No visible cross-reactivity with normal organs was observed.
Keywords: Antibody; Codrituzumab; Glypican; Hepatocellular; I-124.
Conflict of interest statement
Ethics approval and consent to participate
This study was reviewed and approved by MSK’s IRB, and all patients provided verbal and written informed consent.
Consent for publication
N/A.
Competing interests
Two authors, Norihisa Ohishi and Toshihiko Ohtomo, are members of Chugai Pharmaceutical Co., Ltd. The rights to the antibody are held by Hoffmann-La Roche, Inc., which funded the trial. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–624. doi: 10.1093/annonc/mdv577. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

