A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer
- PMID: 29508246
- PMCID: PMC6050986
- DOI: 10.1007/s10637-018-0578-9
A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer
Abstract
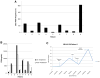
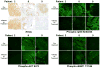
Background MLN0128 is a first-in-class, dual mTOR inhibitor with potential to outperform standard rapalogs through inhibition of TORC1 and TORC2. This phase II study was designed to assess antitumor activity of MLN0128 in metastatic castration-resistant prostate cancer (mCRPC). Methods Eligible patients had mCRPC previously treated with abiraterone acetate and/or enzalutamide. Five patients started MLN0128 at 5 mg once daily, subsequently dose reduced to 4 mg because of toxicity. Four subsequent patients started MLN0128 at 4 mg daily. Primary endpoint was progression-free survival at 6 months. Results Nine patients were enrolled and median time on treatment was 11 weeks (range: 3-30). Best response was stable disease. All patients had a rise in PSA on treatment, with a median 159% increase from baseline (range: 12-620%). Median baseline circulating tumor cell count was 1 cell/mL (range: 0-40); none had a decrease in cell count posttreatment. Grade ≤ 2 adverse events included fatigue, anorexia, and rash. The most common serious adverse events were grade 3 dyspnea and maculopapular rash. Eight patients discontinued treatment early because of radiographic progression (n = 1), grade 3 toxicity (n = 5), or investigator discretion (n = 2). Four patients had immediate PSA decline following drug discontinuation, suggesting MLN0128 could cause compensatory increase of androgen receptor (AR) activity. Correlative studies of pretreatment and posttreatment biopsy specimens revealed limited inhibition of AKT phosphorylation, 4EBP1 phosphorylation, and eIF4E activity. Conclusions Clinical efficacy of MLN0128 in mCRPC was limited likely due to dose reductions secondary to toxicity, PSA kinetics suggesting AR activation resulting from mTOR inhibition, and poor inhibition of mTOR signaling targets.
Keywords: MLN0128; Prostate cancer; mTOR.
Figures



Similar articles
-
A Phase I Study of Abiraterone Acetate Combined with BEZ235, a Dual PI3K/mTOR Inhibitor, in Metastatic Castration Resistant Prostate Cancer.Oncologist. 2017 May;22(5):503-e43. doi: 10.1634/theoncologist.2016-0432. Epub 2017 Mar 17. Oncologist. 2017. PMID: 28314838 Free PMC article. Clinical Trial.
-
Phase I/II study evaluating the safety and clinical efficacy of temsirolimus and bevacizumab in patients with chemotherapy refractory metastatic castration-resistant prostate cancer.Invest New Drugs. 2019 Apr;37(2):331-337. doi: 10.1007/s10637-018-0687-5. Epub 2018 Nov 7. Invest New Drugs. 2019. PMID: 30402678 Clinical Trial.
-
Preclinical trial of a new dual mTOR inhibitor, MLN0128, using renal cell carcinoma tumorgrafts.Int J Cancer. 2014 May 15;134(10):2322-9. doi: 10.1002/ijc.28579. Epub 2013 Nov 18. Int J Cancer. 2014. PMID: 24243565 Free PMC article.
-
A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer.Clin Genitourin Cancer. 2013 Dec;11(4):397-406. doi: 10.1016/j.clgc.2013.05.007. Epub 2013 Jul 3. Clin Genitourin Cancer. 2013. PMID: 23830964 Clinical Trial.
-
A Phase I Trial of IGF-1R Inhibitor Cixutumumab and mTOR Inhibitor Temsirolimus in Metastatic Castration-resistant Prostate Cancer.Clin Genitourin Cancer. 2020 Jun;18(3):171-178.e2. doi: 10.1016/j.clgc.2019.10.013. Epub 2020 Jan 11. Clin Genitourin Cancer. 2020. PMID: 32057715 Free PMC article. Clinical Trial.
Cited by
-
The androgen receptor regulates a druggable translational regulon in advanced prostate cancer.Sci Transl Med. 2019 Jul 31;11(503):eaaw4993. doi: 10.1126/scitranslmed.aaw4993. Sci Transl Med. 2019. PMID: 31366581 Free PMC article.
-
Cancer immune evasion through KRAS and PD-L1 and potential therapeutic interventions.Cell Commun Signal. 2023 Mar 2;21(1):45. doi: 10.1186/s12964-023-01063-x. Cell Commun Signal. 2023. PMID: 36864508 Free PMC article. Review.
-
eIF4E Phosphorylation in Prostate Cancer.Neoplasia. 2018 Jun;20(6):563-573. doi: 10.1016/j.neo.2018.04.003. Epub 2018 May 4. Neoplasia. 2018. PMID: 29730477 Free PMC article. Review.
-
A screening of growth inhibitory activity of Iranian medicinal plants on prostate cancer cell lines.Biomedicine (Taipei). 2018 Jun;8(2):8. doi: 10.1051/bmdcn/2018080208. Epub 2018 May 28. Biomedicine (Taipei). 2018. PMID: 29806586 Free PMC article.
-
The Role of ERα and ERβ in Castration-Resistant Prostate Cancer and Current Therapeutic Approaches.Biomedicines. 2023 Mar 9;11(3):826. doi: 10.3390/biomedicines11030826. Biomedicines. 2023. PMID: 36979805 Free PMC article. Review.
References
-
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.05.026. - DOI - PMC - PubMed
-
- Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, Kozma SC, Thomas G, Cordon-Cardo C, Pandolfi PP. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal. 2009 doi: 10.1126/scisignal.2000189. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- n/a/Prostate Cancer Foundation (US)/International
- 1K08CA175154-01/CA/NCI NIH HHS/United States
- P50 CA092629/CA/NCI NIH HHS/United States
- P50CA092629/CA/NCI NIH HHS/United States
- P30CA008748/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- n/a/American Association for Cancer Research (US)/International
- K08 CA175154/CA/NCI NIH HHS/United States
- n/a/American Society of Clinical Oncology (US)/International
- W81XWH-12-PCRP-TIA/Congressionally Directed Medical Research Programs (US)/International
- R01 CA193837/CA/NCI NIH HHS/United States
- n/a/Millenium Pharmaceuticals/International
- T32CA009515/CA/NCI NIH HHS/United States
- n/a/V Foundation for Cancer Research (US)/International
- R01 CA208100/CA/NCI NIH HHS/United States
- n/a/Burroughs Wellcome Fund (US)/International
- T32 CA009515/CA/NCI NIH HHS/United States
- R01 CA182503/CA/NCI NIH HHS/United States
- 1R01 CA182503-01A1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

