A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer
- PMID: 29508246
- PMCID: PMC6050986
- DOI: 10.1007/s10637-018-0578-9
A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer
Abstract
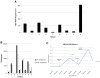
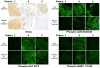
Background MLN0128 is a first-in-class, dual mTOR inhibitor with potential to outperform standard rapalogs through inhibition of TORC1 and TORC2. This phase II study was designed to assess antitumor activity of MLN0128 in metastatic castration-resistant prostate cancer (mCRPC). Methods Eligible patients had mCRPC previously treated with abiraterone acetate and/or enzalutamide. Five patients started MLN0128 at 5 mg once daily, subsequently dose reduced to 4 mg because of toxicity. Four subsequent patients started MLN0128 at 4 mg daily. Primary endpoint was progression-free survival at 6 months. Results Nine patients were enrolled and median time on treatment was 11 weeks (range: 3-30). Best response was stable disease. All patients had a rise in PSA on treatment, with a median 159% increase from baseline (range: 12-620%). Median baseline circulating tumor cell count was 1 cell/mL (range: 0-40); none had a decrease in cell count posttreatment. Grade ≤ 2 adverse events included fatigue, anorexia, and rash. The most common serious adverse events were grade 3 dyspnea and maculopapular rash. Eight patients discontinued treatment early because of radiographic progression (n = 1), grade 3 toxicity (n = 5), or investigator discretion (n = 2). Four patients had immediate PSA decline following drug discontinuation, suggesting MLN0128 could cause compensatory increase of androgen receptor (AR) activity. Correlative studies of pretreatment and posttreatment biopsy specimens revealed limited inhibition of AKT phosphorylation, 4EBP1 phosphorylation, and eIF4E activity. Conclusions Clinical efficacy of MLN0128 in mCRPC was limited likely due to dose reductions secondary to toxicity, PSA kinetics suggesting AR activation resulting from mTOR inhibition, and poor inhibition of mTOR signaling targets.
Keywords: MLN0128; Prostate cancer; mTOR.
Figures



References
-
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.05.026. - DOI - PMC - PubMed
-
- Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, Kozma SC, Thomas G, Cordon-Cardo C, Pandolfi PP. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal. 2009 doi: 10.1126/scisignal.2000189. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- n/a/Prostate Cancer Foundation (US)/International
- 1K08CA175154-01/CA/NCI NIH HHS/United States
- P50 CA092629/CA/NCI NIH HHS/United States
- P50CA092629/CA/NCI NIH HHS/United States
- P30CA008748/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- n/a/American Association for Cancer Research (US)/International
- K08 CA175154/CA/NCI NIH HHS/United States
- n/a/American Society of Clinical Oncology (US)/International
- W81XWH-12-PCRP-TIA/Congressionally Directed Medical Research Programs (US)/International
- R01 CA193837/CA/NCI NIH HHS/United States
- n/a/Millenium Pharmaceuticals/International
- T32CA009515/CA/NCI NIH HHS/United States
- n/a/V Foundation for Cancer Research (US)/International
- R01 CA208100/CA/NCI NIH HHS/United States
- n/a/Burroughs Wellcome Fund (US)/International
- T32 CA009515/CA/NCI NIH HHS/United States
- R01 CA182503/CA/NCI NIH HHS/United States
- 1R01 CA182503-01A1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

