On-target and direct modulation of alloreactive T cells by a nanoparticle carrying MHC alloantigen, regulatory molecules and CD47 in a murine model of alloskin transplantation
- PMID: 29508634
- PMCID: PMC6058602
- DOI: 10.1080/10717544.2018.1447049
On-target and direct modulation of alloreactive T cells by a nanoparticle carrying MHC alloantigen, regulatory molecules and CD47 in a murine model of alloskin transplantation
Abstract
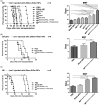
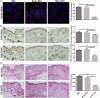
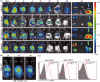
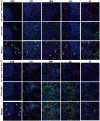
Biomimetic nanoparticles have been reported as immune modulators in autoimmune diseases and allograft rejections by numerous researchers. However, most of the therapeutics carrying antigens, toxins or cytokines underlay the mechanism of antigen presentation by cellular uptake of NPs through pinocytosis and phagocytosis. Few researches focus on the direct and antigen-specific modulation on T cells by NPs and combined use of multiple regulatory molecules. Here, polylactic-co-glycolic acid nanoparticles (PLGA-NPs) were fabricated as scaffold to cocoupling H-2Kb-Ig dimer, anti-Fas mAb, PD-L1-Fc, TGF-β and CD47-Fc for the generation of alloantigen-presenting and tolerance-inducing NPs, termed killer NPs and followed by i.v. injection into a single MHC-mismatched murine model of alloskin transplantation. Three infusions prolonged alloskin graft survival for 45 days; depleted most of H-2Kb alloreactive CD8+ T cells in peripheral blood, spleen and local graft, in an antigen-specific manner. The killer NPs circulated throughout vasculature into various organs and local allograft, with a retention time up to 30 h. They made contacts with CD8+ T cells to facilitate vigorous apoptosis, inhibit the activation and proliferation of alloreactive CD8+ T cells and induce regulatory T cells in secondary lymphoid organs, with the greatly minimized uptake by phagocytes. More importantly, the impairment of host overall immune function and visible organ toxicity were not found. Our results provide the first experimental evidence for the direct and on-target modulation on alloreactive T cells by the biodegradable 200-nm killer NPs via co-presentation of alloantigen and multiple regulatory molecules, thus suggest a novel antigen-specific immune modulator for allograft rejections.
Keywords: Alloskin transplantation; alloreactive T cells; biomimetic nanoparticles; immunotherapy; major histocompatibility complex.
Figures


References
-
- Brandhorst G, Weigand S, Eberle C, et al. (2013). CD4+ immune response as a potential biomarker of patient reported inflammatory bowel disease (IBD) activity. Clin Chim Acta 421:31–3. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
