Pedunculopontine Nucleus Cholinergic Deficiency in Cervical Dystonia
- PMID: 29508906
- PMCID: PMC7299544
- DOI: 10.1002/mds.27358
Pedunculopontine Nucleus Cholinergic Deficiency in Cervical Dystonia
Abstract
Background: The etiology of cervical dystonia is unknown. Cholinergic abnormalities have been identified in dystonia animal models and human imaging studies. Some animal models have cholinergic neuronal loss in the striatum and increased acetylcholinesterase activity in the pedunculopontine nucleus.
Objectives: The objective of this study was to determine the presence of cholinergic abnormalities in the putamen and pedunculopontine nucleus in cervical dystonia human brain donors.
Methods: Formalin-fixed brain tissues were obtained from 8 cervical dystonia and 7 age-matched control brains (controls). Pedunculopontine nucleus was available in only 6 cervical dystonia and 5 controls. Neurodegeneration was evaluated pathologically in the putamen, pedunculopontine nucleus, and other regions. Cholinergic neurons were detected using choline acetyltransferase immunohistochemistry in the putamen and pedunculopontine nucleus. Putaminal cholinergic neurons were quantified. A total of 6 cervical dystonia patients and 6 age-matched healthy controls underwent diffusion tensor imaging to determine if there were white matter microstructural abnormalities around the pedunculopontine nucleus.
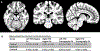
Results: Decreased or absent choline acetyltransferase staining was identified in all 6 pedunculopontine nucleus samples in cervical dystonia. In contrast, strong choline acetyltransferase staining was present in 4 of 5 pedunculopontine nucleus controls. There were no differences in pedunculopontine nucleus diffusion tensor imaging between cervical dystonia and healthy controls. There was no difference in numbers of putaminal cholinergic neurons between cervical dystonia and controls.
Conclusions: Our findings suggest that pedunculopontine nucleus choline acetyltransferase deficiency represents a functional cholinergic deficit in cervical dystonia. Structural lesions and confounding neurodegenerative processes were excluded by absence of neuronal loss, gliosis, diffusion tensor imaging abnormalities, and beta-amyloid, tau, and alpha-synuclein pathologies. © 2018 International Parkinson and Movement Disorder Society.
Keywords: MRI; acetylcholine; cervical dystonia; diffusion tensor imaging; neuropathology.
© 2018 International Parkinson and Movement Disorder Society.
Figures


Similar articles
-
Pedunculopontine cell loss and protein aggregation direct microglia activation in parkinsonian rats.Brain Struct Funct. 2016 May;221(4):2319-41. doi: 10.1007/s00429-015-1045-4. Epub 2015 May 20. Brain Struct Funct. 2016. PMID: 25989851
-
A non-cholinergic neuronal loss in the pedunculopontine nucleus of toxin-evoked parkinsonian rats.Exp Neurol. 2013 Oct;248:213-23. doi: 10.1016/j.expneurol.2013.06.008. Epub 2013 Jun 14. Exp Neurol. 2013. PMID: 23769975
-
Pedunculopontine cholinergic cell loss in hallucinating Parkinson disease patients but not in dementia with Lewy bodies patients.J Neuropathol Exp Neurol. 2013 Dec;72(12):1162-70. doi: 10.1097/NEN.0000000000000014. J Neuropathol Exp Neurol. 2013. PMID: 24226265
-
Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post-mortem immunocytochemistry and in vivo PET imaging with [¹⁸F]fluoroethoxybenzovesamicol.Behav Brain Res. 2015 Feb 1;278:107-14. doi: 10.1016/j.bbr.2014.09.021. Epub 2014 Sep 22. Behav Brain Res. 2015. PMID: 25257103
-
Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system.Pathol Int. 1999 Nov;49(11):921-37. doi: 10.1046/j.1440-1827.1999.00977.x. Pathol Int. 1999. PMID: 10594838 Review.
Cited by
-
Dystonia and the pedunculopontine nucleus: Current evidences and potential mechanisms.Front Neurol. 2022 Nov 23;13:1065163. doi: 10.3389/fneur.2022.1065163. eCollection 2022. Front Neurol. 2022. PMID: 36504662 Free PMC article. Review.
-
Attention impairment in patients with cervical dystonia: An attention network test study.Front Psychol. 2022 Aug 4;13:952567. doi: 10.3389/fpsyg.2022.952567. eCollection 2022. Front Psychol. 2022. PMID: 35992456 Free PMC article.
-
Roles of the M4 acetylcholine receptor in the basal ganglia and the treatment of movement disorders.Mov Disord. 2019 Aug;34(8):1089-1099. doi: 10.1002/mds.27740. Epub 2019 Jun 18. Mov Disord. 2019. PMID: 31211471 Free PMC article. Review.
-
A cell autonomous torsinA requirement for cholinergic neuron survival and motor control.Elife. 2018 Aug 17;7:e36691. doi: 10.7554/eLife.36691. Elife. 2018. PMID: 30117805 Free PMC article.
-
Potential interactions between cerebellar dysfunction and sleep disturbances in dystonia.Dystonia. 2022 Oct;1:10691. doi: 10.3389/dyst.2022.10691. Epub 2022 Oct 4. Dystonia. 2022. PMID: 37065094 Free PMC article.
References
-
- Nutt JG, Muenter MD, Aronson A, Kurland LT, Melton LJ. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3:188–194. - PubMed
-
- Pappas SS, Darr K, Holley SM, Cepeda C, Mabrouk OS, Wong J-MT, LeWitt TM, Paudel R, Houlden H, Kennedy RT, Levine MS, Dauer WT, Albin R, Cross D, Cornblath W, Wald J, Wernette K, Frey K, Minoshima S, Alexander G, DeLong M, Strick P, Berke J, Bressman S, Sabatti C, Raymond D, Leon D de, Klein C, Kramer P, Brin M, Fahn S, Breakefield X, Ozelius L, Risch N, Burke R, Fahn S, Marsden C, Burke R, Karanas A, Carbon M, Argyelan M, Ghilardi M, Mattis P, Dhawan V, Bressman S, Eidelberg D, Cepeda C, Galvan L, Holley S, Rao S, Andre V, Botelho E, Chen J, Watson J, Deisseroth K, Levine M, Chen P, Burdette A, Porter J, Ricketts J, Fox S, Nery F, Hewett J, Berkowitz L, Breakefield X, Caldwell K, Caldwell G, Chesselet M, Plotkin J, Wu N, Levine M, Dauer W, Jaunarajs KE, Bonsi P, Chesselet M, Standaert D, Pisani A, Gage G, Stoetzner C, Wiltschko A, Berke J, Geneser F, Gerace L, Gittis A, Leventhal D, Fensterheim B, Pettibone J, Berke J, Kreitzer A, Goodchild R, Kim C, Dauer W, Grundmann K, Glockle N, Martella G, Sciamanna G, Hauser T, Yu L, et al. Forebrain deletion of the dystonia protein torsinA causes dystonic-like movements and loss of striatal cholinergic neurons. Elife. 2015;4:e08352. - PMC - PubMed
-
- Clément C, Lalonde R, Strazielle C. Acetylcholinesterase activity in the brain of dystonia musculorum (Dst(dt-J)) mutant mice. Neurosci Res. 2012;72:79–86. - PubMed
-
- Ikeda K, Yanagihashi M, Sawada M, Hanashiro S, Kawabe K, Iwasaki Y. Donepezil-induced cervical dystonia in Alzheimer’s disease: a case report and literature review of dystonia due to cholinesterase inhibitors. Intern Med. 2014;53:1007–1010. http://www.ncbi.nlm.nih.gov/pubmed/24785894. Accessed November 24, 2016. - PubMed
-
- Albin RL, Cross D, Cornblath WT, Wald JA, Wernette K, Frey KA, Minoshima S. Diminished striatal [123I]iodobenzovesamicol binding in idiopathic cervical dystonia. Ann Neurol. 2003;53:528–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

