A Selective Phosphodiesterase 10A Inhibitor Reduces L-Dopa-Induced Dyskinesias in Parkinsonian Monkeys
- PMID: 29508924
- PMCID: PMC5992016
- DOI: 10.1002/mds.27341
A Selective Phosphodiesterase 10A Inhibitor Reduces L-Dopa-Induced Dyskinesias in Parkinsonian Monkeys
Abstract
Background: Phosphodiesterase 10A is a member of the phosphodiesterase family whose brain expression is restricted to the striatum. Phosphodiesterase 10A regulates cyclic adenosine monophosphate and cyclic guanosine monophosphate, which mediate responses to dopamine receptor activation, and the levels of these cyclic nucleotides are decreased in experimental models of l-dopa-induced dyskinesia. The elevation of cyclic adenosine monophosphate/cyclic guanosine monophosphate levels by phosphodiesterase 10A inhibition may thus be targeted to reduce l-dopa-induced dyskinesia.
Objectives: The present study was aimed at determining the potential antidyskinetic effects of phosphodiesterase 10A inhibitors in a primate model of Parkinson's disease (PD). The experiments performed in this model were also intended to provide translational data for the design of future clinical trials.
Methods: Five MPTP-treated macaques with advanced parkinsonism and reproducible l-dopa-induced dyskinesia were used. MR1916, a selective phosphodiesterase 10A inhibitor, at doses 0.0015 to 0.05 mg/kg, subcutaneously, or its vehicle (control test) was coadministered with l-dopa methyl ester acutely (predetermined optimal and suboptimal subcutaneous doses) and oral l-dopa chronically as daily treatment for 5 weeks. Standardized scales were used to assess motor disability and l-dopa-induced dyskinesia by blinded examiners. Pharmacokinetics was also examined.
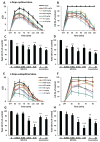
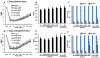
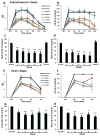
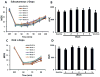
Results: MR1916 consistently reduced l-dopa-induced dyskinesia in acute tests of l-dopa optimal and suboptimal doses. Significant effects were present with every MR1916 dose tested, but the most effective was 0.015 mg/kg. None of the MR1916 doses tested affected the antiparkinsonian action of l-dopa at the optimal dose. The anti-l-dopa-induced dyskinesia effect of MR1916 (0.015 mg/kg, subcutaneously) was sustained with chronic administration, indicating that tolerance did not develop over the 5-week treatment. No adverse effects were observed after MR1916 administration acutely or chronically.
Conclusions: Results show that regulation of striatal cyclic nucleotides by phosphodiesterase 10A inhibition could be a useful therapeutic approach for l-dopa-induced dyskinesia, and therefore data support further studies of selective phosphodiesterase 10A inhibitors for PD therapy. © 2018 International Parkinson and Movement Disorder Society.
Keywords: PDE10A inhibitor; cyclic nucleotides; l-dopa-induced dyskinesia; nonhuman primate models; striatum.
© 2018 International Parkinson and Movement Disorder Society.
Figures
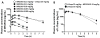
Similar articles
-
Ameliorative effects of a phosphodiesterase 10A inhibitor, MR1916 on L-DOPA-induced dyskinesia in parkinsonian rats.Pharmacol Rep. 2020 Apr;72(2):443-448. doi: 10.1007/s43440-020-00060-y. Epub 2020 Mar 6. Pharmacol Rep. 2020. PMID: 32144743
-
Low doses of sarizotan reduce dyskinesias and maintain antiparkinsonian efficacy of L-Dopa in parkinsonian monkeys.Parkinsonism Relat Disord. 2009 Jul;15(6):445-52. doi: 10.1016/j.parkreldis.2008.11.001. Epub 2009 Feb 3. Parkinsonism Relat Disord. 2009. PMID: 19196540
-
AQW051, a novel and selective nicotinic acetylcholine receptor α7 partial agonist, reduces l-Dopa-induced dyskinesias and extends the duration of l-Dopa effects in parkinsonian monkeys.Parkinsonism Relat Disord. 2014 Nov;20(11):1119-23. doi: 10.1016/j.parkreldis.2014.05.007. Epub 2014 May 22. Parkinsonism Relat Disord. 2014. PMID: 25172125
-
Animal models of l-dopa-induced dyskinesia in Parkinson's disease.Mov Disord. 2018 Jul;33(6):889-899. doi: 10.1002/mds.27337. Epub 2018 Feb 28. Mov Disord. 2018. PMID: 29488257 Review.
-
Translating A2A antagonist KW6002 from animal models to parkinsonian patients.Neurology. 2003 Dec 9;61(11 Suppl 6):S107-11. doi: 10.1212/01.wnl.0000095223.08711.48. Neurology. 2003. PMID: 14663022 Review.
Cited by
-
Targeting Striatal Glutamate and Phosphodiesterases to Control L-DOPA-Induced Dyskinesia.Cells. 2023 Nov 30;12(23):2754. doi: 10.3390/cells12232754. Cells. 2023. PMID: 38067182 Free PMC article. Review.
-
Phosphodiesterase 10A (PDE10A): Regulator of Dopamine Agonist-Induced Gene Expression in the Striatum.Cells. 2022 Jul 16;11(14):2214. doi: 10.3390/cells11142214. Cells. 2022. PMID: 35883657 Free PMC article.
-
Phosphodiesterase 10A Inhibition Modulates the Corticostriatal Activity and L-DOPA-Induced Dyskinesia.Pharmaceuticals (Basel). 2022 Jul 30;15(8):947. doi: 10.3390/ph15080947. Pharmaceuticals (Basel). 2022. PMID: 36015095 Free PMC article.
-
An insight into reactivity and bioactivity properties of quorum sensing peptides against PDE10A: a computational peptidology approach.J Mol Model. 2022 Jul 5;28(8):209. doi: 10.1007/s00894-022-05176-x. J Mol Model. 2022. PMID: 35789297
-
Riluzole Administration to Rats with Levodopa-Induced Dyskinesia Leads to Loss of DNA Methylation in Neuronal Genes.Cells. 2021 Jun 9;10(6):1442. doi: 10.3390/cells10061442. Cells. 2021. PMID: 34207710 Free PMC article.
References
-
- Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–677. - PubMed
-
- Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50:1323–1326. - PubMed
-
- Snow BJ, Macdonald L, McAuley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2000;23:82–5. - PubMed
-
- Herzog J, Pinsker M, Wasner M, et al. Stimulation of subthalamic fibre tracts reduces dyskinesias in STN-DBS. Mov Disord. 2007;22:679–684. - PubMed
-
- Hamani C, Neimat J, Lozano AM. Deep brain stimulation for the treatment of Parkinson’s disease. J Neural Transm Suppl. 2006:393–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

