Myeloid-specific deletion of Zfp36 protects against insulin resistance and fatty liver in diet-induced obese mice
- PMID: 29509432
- PMCID: PMC6230714
- DOI: 10.1152/ajpendo.00224.2017
Myeloid-specific deletion of Zfp36 protects against insulin resistance and fatty liver in diet-induced obese mice
Abstract
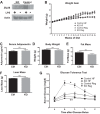
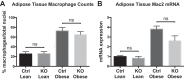
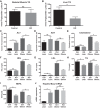
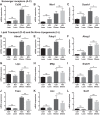
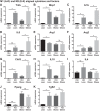
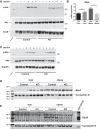
Obesity is associated with adipose tissue inflammation that contributes to insulin resistance. Zinc finger protein 36 (Zfp36) is an mRNA-binding protein that reduces inflammation by binding to cytokine transcripts and promoting their degradation. We hypothesized that myeloid-specific deficiency of Zfp36 would lead to increased adipose tissue inflammation and reduced insulin sensitivity in diet-induced obese mice. As expected, wild-type (Control) mice became obese and diabetic on a high-fat diet, and obese mice with myeloid-specific loss of Zfp36 [knockout (KO)] demonstrated increased adipose tissue and liver cytokine mRNA expression compared with Control mice. Unexpectedly, in glucose tolerance testing and hyperinsulinemic-euglycemic clamp studies, myeloid Zfp36 KO mice demonstrated improved insulin sensitivity compared with Control mice. Obese KO and Control mice had similar macrophage infiltration of the adipose depots and similar peripheral cytokine levels, but lean and obese KO mice demonstrated increased Kupffer cell (KC; the hepatic macrophage)-expressed Mac2 compared with lean Control mice. Insulin resistance in obese Control mice was associated with enhanced Zfp36 expression in KCs. Compared with Control mice, KO mice demonstrated increased hepatic mRNA expression of a multitude of classical (M1) inflammatory cytokines/chemokines, and this M1-inflammatory hepatic milieu was associated with enhanced nuclear localization of IKKβ and the p65 subunit of NF-κB. Our data confirm the important role of innate immune cells in regulating hepatic insulin sensitivity and lipid metabolism, challenge-prevailing models in which M1 inflammatory responses predict insulin resistance, and indicate that myeloid-expressed Zfp36 modulates the response to insulin in mice.
Keywords: Zfp36; diabetes; inflammation; insulin; liver; obesity.
Figures





References
-
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab 13: 401–412, 2011. doi:10.1016/j.cmet.2011.02.010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

