Developmental origins of nonalcoholic fatty liver disease as a risk factor for exaggerated metabolic and cardiovascular-renal disease
- PMID: 29509436
- PMCID: PMC6293166
- DOI: 10.1152/ajpendo.00394.2017
Developmental origins of nonalcoholic fatty liver disease as a risk factor for exaggerated metabolic and cardiovascular-renal disease
Abstract
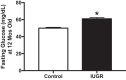
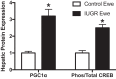
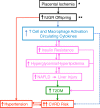
Intrauterine growth restriction (IUGR) is linked to increased risk for chronic disease. Placental ischemia and insufficiency in the mother are implicated in predisposing IUGR offspring to metabolic dysfunction, including hypertension, insulin resistance, abnormalities in glucose homeostasis, and nonalcoholic fatty liver disease (NAFLD). It is unclear whether these metabolic disturbances contribute to the developmental origins of exaggerated cardiovascular-renal disease (CVRD) risk accompanying IUGR. IUGR impacts the pancreas, adipose tissue, and liver, which are hypothesized to program for hepatic insulin resistance and subsequent NAFLD. NAFLD is projected to become the major cause of chronic liver disease and contributor to uncontrolled type 2 diabetes mellitus, which is a leading cause of chronic kidney disease. While NAFLD is increased in experimental models of IUGR, lacking is a full comprehension of the mechanisms responsible for programming of NAFLD and whether this potentiates susceptibility to liver injury. The use of well-established and clinically relevant rodent models, which mimic the clinical characteristics of IUGR, metabolic disturbances, and increased blood pressure in the offspring, will permit investigation into mechanisms linking adverse influences during early life and later chronic health. The purpose of this review is to propose mechanisms, including those proinflammatory in nature, whereby IUGR exacerbates the pathogenesis of NAFLD and how these adverse programmed outcomes contribute to exaggerated CVRD risk. Understanding the etiology of the developmental origins of chronic disease will allow investigators to uncover treatment strategies to intervene in the mother and her offspring to halt the increasing prevalence of metabolic dysfunction and CVRD.
Keywords: hypertension; inflammation; insulin resistance; placental ischemia; steatosis.
Figures

Similar articles
-
Nonalcoholic Fatty Liver Disease (NAFLD)-A New Cardiovascular Risk Factor in Peritoneal Dialysis Patients.Perit Dial Int. 2016 Jul-Aug;36(4):427-32. doi: 10.3747/pdi.2014.00223. Epub 2015 Oct 16. Perit Dial Int. 2016. PMID: 26475841 Free PMC article.
-
Placental insufficiency contributes to fatty acid metabolism alterations in aged female mouse offspring.Am J Physiol Regul Integr Comp Physiol. 2018 Dec 1;315(6):R1107-R1114. doi: 10.1152/ajpregu.00420.2017. Epub 2018 Sep 12. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 30207754
-
Nonalcoholic fatty liver disease as a multi-systemic disease.World J Gastroenterol. 2016 Apr 28;22(16):4079-90. doi: 10.3748/wjg.v22.i16.4079. World J Gastroenterol. 2016. PMID: 27122660 Free PMC article. Review.
-
Systemic Complications of Nonalcoholic Fatty Liver Disease: When the Liver Is Not an Innocent Bystander.Semin Liver Dis. 2015 Aug;35(3):236-49. doi: 10.1055/s-0035-1562944. Epub 2015 Sep 17. Semin Liver Dis. 2015. PMID: 26378641 Review.
-
Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review.J Am Coll Cardiol. 2019 Mar 5;73(8):948-963. doi: 10.1016/j.jacc.2018.11.050. J Am Coll Cardiol. 2019. PMID: 30819364 Review.
Cited by
-
Liver Proteome Profile of Growth Restricted and Appropriately Grown Newborn Wistar Rats Associated With Maternal Undernutrition.Front Endocrinol (Lausanne). 2021 May 28;12:684220. doi: 10.3389/fendo.2021.684220. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34127923 Free PMC article.
-
Adrenergic receptor blockade attenuates placental ischemia-induced hypertension.Physiol Rep. 2018 Sep;6(17):e13814. doi: 10.14814/phy2.13814. Physiol Rep. 2018. PMID: 30229567 Free PMC article.
-
The association between perinatal factors and cardiometabolic risk factors in children and adolescents with overweight or obesity: A retrospective two-cohort study.PLoS Med. 2023 Jan 13;20(1):e1004165. doi: 10.1371/journal.pmed.1004165. eCollection 2023 Jan. PLoS Med. 2023. PMID: 36638094 Free PMC article.
-
Genetics, epigenetics and transgenerational transmission of obesity in children.Front Endocrinol (Lausanne). 2022 Nov 14;13:1006008. doi: 10.3389/fendo.2022.1006008. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36452324 Free PMC article. Review.
-
Alterations in DNA methylation associate with fatty liver and metabolic abnormalities in a multi-ethnic cohort of pre-teenage children.Epigenetics. 2022 Nov;17(11):1446-1461. doi: 10.1080/15592294.2022.2039850. Epub 2022 Feb 21. Epigenetics. 2022. PMID: 35188871 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

