A PET imaging agent for evaluating PARP-1 expression in ovarian cancer
- PMID: 29509546
- PMCID: PMC5919879
- DOI: 10.1172/JCI97992
A PET imaging agent for evaluating PARP-1 expression in ovarian cancer
Abstract
Background: Poly(ADP-ribose) polymerase (PARP) inhibitors are effective in a broad population of patients with ovarian cancer; however, resistance caused by low enzyme expression of the drug target PARP-1 remains to be clinically evaluated in this context. We hypothesize that PARP-1 expression is variable in ovarian cancer and can be quantified in primary and metastatic disease using a novel PET imaging agent.
Methods: We used a translational approach to describe the significance of PET imaging of PARP-1 in ovarian cancer. First, we produced PARP1-KO ovarian cancer cell lines using CRISPR/Cas9 gene editing to test the loss of PARP-1 as a resistance mechanism to all clinically used PARP inhibitors. Next, we performed preclinical microPET imaging studies using ovarian cancer patient-derived xenografts in mouse models. Finally, in a phase I PET imaging clinical trial we explored PET imaging as a regional marker of PARP-1 expression in primary and metastatic disease through correlative tissue histology.
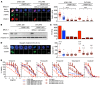
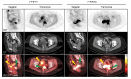
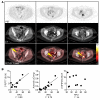
Results: We found that deletion of PARP1 causes resistance to all PARP inhibitors in vitro, and microPET imaging provides proof of concept as an approach to quantify PARP-1 in vivo. Clinically, we observed a spectrum of standard uptake values (SUVs) ranging from 2-12 for PARP-1 in tumors. In addition, we found a positive correlation between PET SUVs and fluorescent immunohistochemistry for PARP-1 (r2 = 0.60).
Conclusion: This work confirms the translational potential of a PARP-1 PET imaging agent and supports future clinical trials to test PARP-1 expression as a method to stratify patients for PARP inhibitor therapy.
Trial registration: Clinicaltrials.gov NCT02637934.
Funding: Research reported in this publication was supported by the Department of Defense OC160269, a Basser Center team science grant, NIH National Cancer Institute R01CA174904, a Department of Energy training grant DE-SC0012476, Abramson Cancer Center Radiation Oncology pilot grants, the Marsha Rivkin Foundation, Kaleidoscope of Hope Foundation, and Paul Calabresi K12 Career Development Award 5K12CA076931.
Keywords: Diagnostic imaging; Molecular biology; Oncology; Pharmacology; Therapeutics.
Conflict of interest statement
Figures




Comment in
-
The evolving landscape of predictive biomarkers of response to PARP inhibitors.J Clin Invest. 2018 May 1;128(5):1727-1730. doi: 10.1172/JCI120388. Epub 2018 Apr 16. J Clin Invest. 2018. PMID: 29664016 Free PMC article.
References
-
- Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5(11):1137–1154. doi: 10.1158/2159-8290.CD-15-0714. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

