Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II
- PMID: 29509568
- PMCID: PMC5959265
- DOI: 10.1097/CCM.0000000000003092
Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II
Erratum in
-
Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II: Erratum.Crit Care Med. 2018 Aug;46(8):e824. doi: 10.1097/CCM.0000000000003226. Crit Care Med. 2018. PMID: 30004987 Free PMC article. No abstract available.
Abstract
Objective: Acute kidney injury requiring renal replacement therapy in severe vasodilatory shock is associated with an unfavorable prognosis. Angiotensin II treatment may help these patients by potentially restoring renal function without decreasing intrarenal oxygenation. We analyzed the impact of angiotensin II on the outcomes of acute kidney injury requiring renal replacement therapy.
Design: Post hoc analysis of the Angiotensin II for the Treatment of High-Output Shock 3 trial.
Setting: ICUs.
Patients: Patients with acute kidney injury treated with renal replacement therapy at initiation of angiotensin II or placebo (n = 45 and n = 60, respectively).
Interventions: IV angiotensin II or placebo.
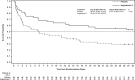
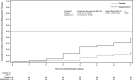
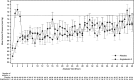
Measurements and main results: Primary end point: survival through day 28; secondary outcomes included renal recovery through day 7 and increase in mean arterial pressure from baseline of ≥ 10 mm Hg or increase to ≥ 75 mm Hg at hour 3. Survival rates through day 28 were 53% (95% CI, 38%-67%) and 30% (95% CI, 19%-41%) in patients treated with angiotensin II and placebo (p = 0.012), respectively. By day 7, 38% (95% CI, 25%-54%) of angiotensin II patients discontinued RRT versus 15% (95% CI, 8%-27%) placebo (p = 0.007). Mean arterial pressure response was achieved in 53% (95% CI, 38%-68%) and 22% (95% CI, 12%-34%) of patients treated with angiotensin II and placebo (p = 0.001), respectively.
Conclusions: In patients with acute kidney injury requiring renal replacement therapy at study drug initiation, 28-day survival and mean arterial pressure response were higher, and rate of renal replacement therapy liberation was greater in the angiotensin II group versus the placebo group. These findings suggest that patients with vasodilatory shock and acute kidney injury requiring renal replacement therapy may preferentially benefit from angiotensin II.
Conflict of interest statement
Shannan Lynch, Jeff Jensen, Stew Kroll, Lakhmir Chawla, and George Tidmarsh are employees of La Jolla Pharmaceutical Company. The Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3) trial was funded and supported by La Jolla Pharmaceutical Company. All other authors participated in the ATHOS-3 trial as investigators and work(ed) at institutions that were funded by La Jolla Pharmaceutical Company in support of the ATHOS-3 trial. Additionally, John R. Prowle has received consultancy fees and speaker honoraria from Nikkiso Europe GmbH and speaker honoraria from Baxter, Inc. Raghavan Murugan was awarded a research grant from La Jolla Pharmaceutical Company, and has received consulting fees from Beckman and Coulter, Inc and Bioporto, Inc. Kianoush Kashani has received a travel grant from La Jolla Pharmaceutical Company for the ATHOS-3 investigator meeting. Marlies Ostermann has received research funding and speaker honoraria from Fresenius Medical Care. Alexander Zarbock has received consulting fees from Astellas and Quark Pharmaceutical; speaker honoraria from Astute Medical, Baxter, Frensenius, and Braun; and grant support from Astute Medical and Quark Pharmaceutical. James Tumlin has received research grant support from La Jolla Pharmaceutical Company. Kevin Finkel will be a member of the La Jolla Pharmaceutical Company Speakers Bureau in 2018. Lawrence Busse has received consulting fees from La Jolla Pharmaceutical Company. Lui Forni has received research funding and honoraria from Fresenius, Baxter Gambro Renal, OrthoClinical Diagnostics, and La Jolla Pharmaceutical Company. Drs. Tumlin, Murugan, Deane, Ham, Szerlip, Prowle, Bihorac, Zarbock, and Bellomo’s institutions received funding from La Jolla Pharmaceuticals. Drs. Tumlin and Kroll disclosed off-label product use of angiotensin II for circulatory shock. Drs. Busse, Forni, and Kroll received funding from La Jolla Pharmaceutical Company. Dr. Szerlip’s institution also received funding from Baylor Research Institute. Dr. Finkel received funding from Alexion Pharmaceuticals (speakers bureau). Dr. Zarbock received funding from Baxter, Astute Medical, Fresenius, Braun, Astellas, and DFG. Dr. Forni also received funding from Ortho Clinical Diagnostics (honoraria). Drs. Lynch, Jensen, Kroll, Chawla, and Tidmarsh disclosed that they are employees of the trial sponsor (La Jolla Pharmaceutical Company). Dr. Tidmarsh disclosed work for hire. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
Comment in
-
Blood Pressure Covariance Not Accounted for in Angiotensin II for the Treatment of High-Output Shock 3 Subgroup Analysis.Crit Care Med. 2019 Mar;47(3):e270-e271. doi: 10.1097/CCM.0000000000003548. Crit Care Med. 2019. PMID: 30768521 No abstract available.
-
The authors reply.Crit Care Med. 2019 Mar;47(3):e271-e272. doi: 10.1097/CCM.0000000000003601. Crit Care Med. 2019. PMID: 30768522 No abstract available.
References
-
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013; 369:1726–1734. - PubMed
-
- De Backer D, Biston P, Devriendt J, et al. SOAP II Investigators: Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789. - PubMed
-
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001; 345:588–595. - PubMed
-
- Chawla LS, Bellomo R, Bihorac A, et al. Acute Disease Quality Initiative Workgroup 16: Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13:241–257. - PubMed
-
- Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 2017; 43:730–749. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

