Electrospun Fibrous Scaffolds for Small-Diameter Blood Vessels: A Review
- PMID: 29509698
- PMCID: PMC5872197
- DOI: 10.3390/membranes8010015
Electrospun Fibrous Scaffolds for Small-Diameter Blood Vessels: A Review
Abstract
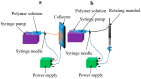
Small-diameter blood vessels (SDBVs) are still a challenging task to prepare due to the occurrence of thrombosis formation, intimal hyperplasia, and aneurysmal dilation. Electrospinning technique, as a promising tissue engineering approach, can fabricate polymer fibrous scaffolds that satisfy requirements on the construction of extracellular matrix (ECM) of native blood vessel and promote the adhesion, proliferation, and growth of cells. In this review, we summarize the polymers that are deployed for the fabrication of SDBVs and classify them into three categories, synthetic polymers, natural polymers, and hybrid polymers. Furthermore, the biomechanical properties and the biological activities of the electrospun SBVs including anti-thrombogenic ability and cell response are discussed. Polymer blends seem to be a strategic way to fabricate SDBVs because it combines both suitable biomechanical properties coming from synthetic polymers and favorable sites to cell attachment coming from natural polymers.
Keywords: SDBVs; anti-thrombogenic agents; electrospinning; fibrous scaffold; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Collins A.J.F. United States renal data system 2011 annual data report: Atlasofchronickidney disease & end stagerenal disease in the United States. Am. J. Kidney Dis. 2012;59:e1–e420. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

