A Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid
- PMID: 29509718
- PMCID: PMC5872069
- DOI: 10.3390/bios8010021
A Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid
Abstract
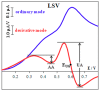
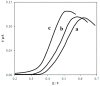
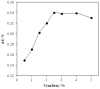
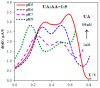
The paper discusses the mechanism of uric acid (UA) electrooxidation occurring on the surface of gold nanoparticles. It has been shown that the electrode process is purely electrochemical, uncomplicated with catalytic stages. The nanoeffects observed as the reduction of overvoltage and increased current of UA oxidation have been described. These nanoeffects are determined by the size of particles and do not depend on the method of particle preparation (citrate and "green" synthesis). The findings of these studies have been used to select a modifier for carbon screen-printed electrode (CSPE). It has been stated that CSPE modified with gold nanoparticles (5 nm) and 2.5% Nafion (Nf) may serve as non-enzymatic sensor for UA determination. The combination of the properties of nanoparticles and Nafion as a molecular sieve at the selected pH 5 phosphate buffer solution has significantly improved the resolution of the sensor compared to unmodified CSPE. A nanostructured sensor has demonstrated good selectivity in determining UA in the presence of ascorbic acid. The detection limit of UA is 0.25 μM. A linear calibration curve has been obtained over a range of 0.5-600 μM. The 2.5%Nf/Au(5nm)/CSPE has been successfully applied to determining UA in blood serum and milk samples. The accuracy and reliability of the obtained results have been confirmed by a good correlation with the enzymatic spectrophotometric analysis (R² = 0.9938) and the "added-found" technique (recovery close to 100%).
Keywords: Nafion; blood serum; carbon screen-printed electrode; gold nanoparticles; milk; nanoeffects; uric acid; ”green” synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Determination of morphine at gold nanoparticles/Nafion® carbon paste modified sensor electrode.Analyst. 2011 Nov 21;136(22):4682-91. doi: 10.1039/c1an15423k. Epub 2011 Aug 30. Analyst. 2011. PMID: 21879032
-
A simple ultrasensitive electrochemical sensor for simultaneous determination of gallic acid and uric acid in human urine and fruit juices based on zirconia-choline chloride-gold nanoparticles-modified carbon paste electrode.Biosens Bioelectron. 2018 Aug 30;114:30-36. doi: 10.1016/j.bios.2018.05.009. Epub 2018 May 8. Biosens Bioelectron. 2018. PMID: 29775856
-
Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor.Anal Biochem. 2010 May 1;400(1):78-88. doi: 10.1016/j.ab.2010.01.001. Epub 2010 Jan 11. Anal Biochem. 2010. PMID: 20064483
-
Advanced design of chemically modified electrodes for the electrochemical analysis of uric acid and xanthine.J Pharm Biomed Anal. 2025 Jan 15;253:116536. doi: 10.1016/j.jpba.2024.116536. Epub 2024 Oct 18. J Pharm Biomed Anal. 2025. PMID: 39476436 Review.
-
Electrochemical sensors for improved detection of paraquat in food samples: A review.Mater Sci Eng C Mater Biol Appl. 2020 Feb;107:110349. doi: 10.1016/j.msec.2019.110349. Epub 2019 Oct 23. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31761239 Review.
Cited by
-
The Effect of the Antioxidant Activity of Plant Extracts on the Properties of Gold Nanoparticles.Nanomaterials (Basel). 2019 Nov 21;9(12):1655. doi: 10.3390/nano9121655. Nanomaterials (Basel). 2019. PMID: 31766367 Free PMC article.
-
Perfluorosulfonic Acid Membranes with Short and Long Side Chains and Their Use in Sensors for the Determination of Markers of Viral Diseases in Saliva.Membranes (Basel). 2023 Jul 27;13(8):701. doi: 10.3390/membranes13080701. Membranes (Basel). 2023. PMID: 37623762 Free PMC article.
-
Fabrication and Optimization of Nafion as a Protective Membrane for TiN-Based pH Sensors.Sensors (Basel). 2023 Feb 20;23(4):2331. doi: 10.3390/s23042331. Sensors (Basel). 2023. PMID: 36850929 Free PMC article.
-
Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles.Materials (Basel). 2018 Apr 27;11(5):687. doi: 10.3390/ma11050687. Materials (Basel). 2018. PMID: 29702583 Free PMC article.
-
Alprazolam Detection Using an Electrochemical Nanobiosensor Based on AuNUs/Fe-Ni@rGO Nanocomposite.Biosensors (Basel). 2022 Oct 31;12(11):945. doi: 10.3390/bios12110945. Biosensors (Basel). 2022. PMID: 36354454 Free PMC article.
References
-
- Wang C., Du J., Wang H., Zou C., Jiang F., Yang P., Du Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B. 2014;204:302–309. doi: 10.1016/j.snb.2014.07.077. - DOI
-
- Zen J.-M., Jou J.-J., Ilangovan G. Selective voltammetric method for uric acid detection using pre-anodized Nafion-coated glassy carbon electrodes. Analyst. 1998;123:1345–1350. doi: 10.1039/a801532e. - DOI
-
- Filik H., Avan A.A., Aydar S. Simultaneous detection of ascorbic acid, dopamine, uric acid and tryptophan with Azure A-interlinked multi-walled carbon nanotube/gold nanoparticles composite modified electrode. Arabian J. Chem. 2016;9:471–480. doi: 10.1016/j.arabjc.2015.01.014. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

