Live-Attenuated Respiratory Syncytial Virus Vaccine Candidate With Deletion of RNA Synthesis Regulatory Protein M2-2 is Highly Immunogenic in Children
- PMID: 29509911
- PMCID: PMC5894092
- DOI: 10.1093/infdis/jiy040
Live-Attenuated Respiratory Syncytial Virus Vaccine Candidate With Deletion of RNA Synthesis Regulatory Protein M2-2 is Highly Immunogenic in Children
Abstract
Background: Live respiratory syncytial virus (RSV) candidate vaccine LIDΔM2-2 is attenuated by deletion of the RSV RNA regulatory protein M2-2, resulting in upregulated viral gene transcription and antigen expression but reduced RNA replication.
Methods: RSV-seronegative children ages 6-24 months received a single intranasal dose of 105 plaque forming units (PFU) of LIDΔM2-2 (n = 20) or placebo (n = 9) (NCT02237209, NCT02040831). RSV serum antibodies, vaccine infectivity, and reactogenicity were assessed. During the following RSV season, participants were monitored for respiratory illness and pre- and post-RSV season serum antibodies.
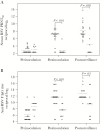
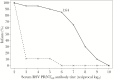
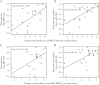
Results: Vaccine virus was shed by 95% of vaccinees (median peak titers of 3.8 log10 PFU/mL by quantitative culture and 6.3 log10 copies/mL by PCR); 90% had ≥4-fold rise in serum neutralizing antibodies. Respiratory symptoms and fever were common in vaccine (95%) and placebo (78%). One vaccinee had grade 2 rhonchi concurrent with vaccine shedding, rhinovirus, and enterovirus. Eight of 19 vaccinees versus 2 of 9 placebo recipients had substantially increased RSV antibody titers after the RSV season without medically attended RSV disease, indicating anamnestic vaccine responses to wild-type RSV without significant illness.
Conclusion: LIDΔM2-2 had excellent infectivity and immunogenicity, encouraging further study of vaccine candidates attenuated by M2-2 deletion.
Clinical trials registration: NCT02237209, NCT02040831.
Figures

Comment in
-
Crafting Live-Attenuated Vaccines Against Respiratory Syncytial Virus.J Infect Dis. 2018 Apr 11;217(9):1335-1337. doi: 10.1093/infdis/jiy038. J Infect Dis. 2018. PMID: 29509928 No abstract available.
References
-
- Karron RA, Black RE. Determining the burden of respiratory syncytial virus disease: the known and the unknown. Lancet 2017; 390:917–18. - PubMed
-
- Groothuis JR, Simoes EA, Levin MJ et al. . Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med 1993; 329:1524–30. - PubMed
-
- Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. American Academy of Pediatrics Committee on Infectious Diseases Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed Elk Grove Village, IL:American Academy of Pediatrics, 2015.
-
- Giersing BK, Vekemans J, Nava S, Kaslow DC, Moorthy V. Report from the World Health Organization’s third Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 8–10th June 2016 [published online ahead of print 2 March, 2017]. Vaccine 2017; doi: 10.1016/j.vaccine.2016.10.090. - PMC - PubMed
-
- Polack FP, Karron RA. The future of respiratory syncytial virus vaccine development. Pediatr Infect Dis J 2004; 23:S65–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

