Live Respiratory Syncytial Virus (RSV) Vaccine Candidate Containing Stabilized Temperature-Sensitivity Mutations Is Highly Attenuated in RSV-Seronegative Infants and Children
- PMID: 29509929
- PMCID: PMC5894088
- DOI: 10.1093/infdis/jiy066
Live Respiratory Syncytial Virus (RSV) Vaccine Candidate Containing Stabilized Temperature-Sensitivity Mutations Is Highly Attenuated in RSV-Seronegative Infants and Children
Abstract
Background: Respiratory syncytial virus (RSV) is the most important viral cause of severe respiratory illness in young children and lacks a vaccine. RSV cold-passage/stabilized 2 (RSVcps2) is a modification of a previously evaluated vaccine candidate in which 2 major attenuating mutations have been stabilized against deattenuation.
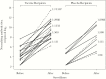
Methods: RSV-seronegative 6-24-month-old children received an intranasal dose of 105.3 plaque-forming units (PFU) of RSVcps2 (n = 34) or placebo (n = 16) (International Maternal Pediatric Adolescent AIDS Clinical Trials protocol P1114 and companion protocol CIR285). RSV serum neutralizing antibody titers before and 56 days after vaccination, vaccine virus infectivity (defined as vaccine virus shedding detectable in nasal wash and/or a ≥4-fold rise in serum antibodies), reactogenicity, and genetic stability were assessed. During the following RSV transmission season, participants were monitored for respiratory illness, with serum antibody titers measured before and after the season.
Results: A total of 85% of vaccinees were infected with RSVcps2 (median peak titer, 0.5 log10 PFU/mL by culture and 2.9 log10 copies/mL by polymerase chain reaction analysis); 77% shed vaccine virus, and 59% developed a ≥4-fold rise in RSV-serum neutralizing antibody titers. Respiratory tract and/or febrile illness occurred at the same rate (50%) in the vaccine and placebo groups. Deattenuation was not detected at either of 2 stabilized mutation sites.
Conclusions: RSVcps2 was well tolerated and moderately immunogenic and had increased genetic stability in 6-24-month-old RSV-seronegative children.
Clinical trials registration: NCT01852266 and NCT01968083.
Figures
References
-
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. - PubMed
-
- Kim HW, Canchola JG, Brandt CD et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. - PubMed
-
- Caballero MT, Jones MH, Karron RA et al. ; RSV & Pediatric Asthma Working Group The impact of respiratory syncytial virus disease prevention on pediatric asthma. Pediatr Infect Dis J 2016; 35:820–2. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

