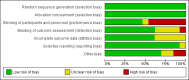
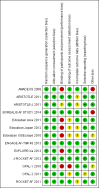
Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation
- PMID: 29509959
- PMCID: PMC6494202
- DOI: 10.1002/14651858.CD008980.pub3
Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation
Abstract
Background: Factor Xa inhibitors and vitamin K antagonists (VKAs) are now recommended in treatment guidelines for preventing stroke and systemic embolic events in people with atrial fibrillation (AF). This is an update of a Cochrane review previously published in 2013.
Objectives: To assess the effectiveness and safety of treatment with factor Xa inhibitors versus VKAs for preventing cerebral or systemic embolic events in people with AF.
Search methods: We searched the trials registers of the Cochrane Stroke Group and the Cochrane Heart Group (September 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (August 2017), MEDLINE (1950 to April 2017), and Embase (1980 to April 2017). We also contacted pharmaceutical companies, authors and sponsors of relevant published trials. We used outcome data from marketing authorisation applications of apixaban, edoxaban and rivaroxaban that were submitted to regulatory authorities in Europe and the USA.
Selection criteria: We included randomised controlled trials (RCTs) that directly compared the effects of long-term treatment (lasting more than four weeks) with factor Xa inhibitors versus VKAs for preventing cerebral and systemic embolism in people with AF.
Data collection and analysis: The primary efficacy outcome was the composite endpoint of all strokes and systemic embolic events. Two review authors independently extracted data, and assessed the quality of the trials and the risk of bias. We calculated a weighted estimate of the typical treatment effect across trials using the odds ratio (OR) with 95% confidence interval (CI) by means of a fixed-effect model. In case of moderate or high heterogeneity of treatment effects, we used a random-effects model to compare the overall treatment effects. We also performed a pre-specified sensitivity analysis excluding any open-label studies.
Main results: We included data from 67,688 participants randomised into 13 RCTs. The included trials directly compared dose-adjusted warfarin with either apixaban, betrixaban, darexaban, edoxaban, idraparinux, idrabiotaparinux, or rivaroxaban. The majority of the included data (approximately 90%) was from apixaban, edoxaban, and rivaroxaban.The composite primary efficacy endpoint of all strokes (both ischaemic and haemorrhagic) and non-central nervous systemic embolic events was reported in all of the included studies. Treatment with a factor Xa inhibitor significantly decreased the number of strokes and systemic embolic events compared with dose-adjusted warfarin in participants with AF (OR 0.89, 95% CI 0.82 to 0.97; 13 studies; 67,477 participants; high-quality evidence).Treatment with a factor Xa inhibitor significantly reduced the number of major bleedings compared with warfarin (OR 0.78, 95% CI 0.73 to 0.84; 13 studies; 67,396 participants; moderate-quality evidence). There was, however, statistically significant and high heterogeneity (I2 = 83%). When we repeated this analysis using a random-effects model, it did not show a statistically significant decrease in the number of major bleedings (OR 0.88, 95% CI 0.66 to 1.17). A pre-specified sensitivity analysis excluding all open-label studies showed that treatment with a factor Xa inhibitor significantly reduced the number of major bleedings compared with warfarin (OR 0.75, 95% CI 0.69 to 0.81), but high heterogeneity was also observed in this analysis (I2 = 72%). The same sensitivity analysis using a random-effects model also showed a statistically significant decrease in the number of major bleedings in participants treated with factor Xa inhibitors (OR 0.76, 95% CI 0.60 to 0.96).Treatment with a factor Xa inhibitor significantly reduced the risk of intracranial haemorrhages (ICHs) compared with warfarin (OR 0.50, 95% CI 0.42 to 0.59; 12 studies; 66,259 participants; high-quality evidence). We observed moderate, but statistically significant heterogeneity (I2 = 55%). The pre-specified sensitivity analysis excluding open-label studies showed that treatment with a factor Xa inhibitor significantly reduced the number of ICHs compared with warfarin (OR 0.47, 95% CI 0.40 to 0.56), with low, non-statistically significant heterogeneity (I2 = 27%).Treatment with a factor Xa inhibitor also significantly reduced the number of all-cause deaths compared with warfarin (OR 0.89, 95% 0.83 to 0.95; 10 studies; 65,624 participants; moderate-quality evidence).
Authors' conclusions: Treatment with factor Xa inhibitors significantly reduced the number of strokes and systemic embolic events compared with warfarin in people with AF. The absolute effect of factor Xa inhibitors compared with warfarin treatment was, however, rather small. Factor Xa inhibitors also reduced the number of ICHs, all-cause deaths and major bleedings compared with warfarin, although the evidence for a reduction in the latter is less robust.
Conflict of interest statement
KBS is currently employed by F. Hoffmann‐La Roche (Roche Norge AS). The data included in this review are based on research which has had no influence or involvement by F. Hoffmann‐La Roche by any means. The views expressed in this review are the personal views of KBS and should not be understood or quoted as being made on behalf of or reflecting the position of F. Hoffmann‐La Roche.
EB chaired a symposium organised by Bayer (entitled “Real World Data”) during the Nordic Stroke Conference in August 2017, after the review had been submitted. He received EUR 900 in compensation for this work. This was in breach of Cochrane's Conflicts of Interest policy, but this has been discussed with the Funding Arbiters, who agreed to allow publication of this updated review.
Figures
Update of
-
Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation.Cochrane Database Syst Rev. 2013 Aug 8;(8):CD008980. doi: 10.1002/14651858.CD008980.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Mar 06;3:CD008980. doi: 10.1002/14651858.CD008980.pub3. PMID: 23925867 Updated.
References
References to studies included in this review
AMADEUS 2008 {published and unpublished data}
-
- The Amadeus Investigators. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open‐label, non‐inferiority trial. Lancet 2008;371:315‐21. - PubMed
ARISTOTLE 2011 {published and unpublished data}
-
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2011;365:981‐92. - PubMed
ARISTOTLE‐J 2011 {published data only (unpublished sought but not used)}
-
- Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non‐valvular atrial fibrillation. Circulation Journal 2011;75:1852‐9. - PubMed
BOREALIS AF STUDY 2014 {published data only}
-
- Buller HR, Halperin J, Hankey GJ, Pillion G, Prins MH, Raskob GE. Comparison of idrabiotaparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: the Borealis‐Atrial Fibrillation Study. Journal of Thrombosis and Haemostasis 2014;12(6):824‐30. - PubMed
Edoxaban Asia 2011 {published data only (unpublished sought but not used)}
-
- Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non‐valvular atrial fibrillation. Thrombosis and Haemostasis 2011;105:535‐45. - PubMed
Edoxaban Japan 2012 {published data only}
-
- Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T, et al. Randomized, multicenter, warfarin‐controlled phase II study of edoxaban in Japanese patients with non‐valvular atrial fibrillation. Circulation Journal 2012;76:1840‐7. - PubMed
Edoxaban US/Europe 2010 {published data only (unpublished sought but not used)}
-
- Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel‐group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thrombosis and Haemostasis 2010;104:633‐41. - PubMed
ENGAGE AF‐TIMI 48 2013 {published and unpublished data}
-
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2013;369:2093‐104. - PubMed
EXPLORE‐Xa 2013 {published data only (unpublished sought but not used)}
-
- Connoly SJ, Eikelboom J, Dorian P, Hohnloser SH, Gretler DD, Sinha U, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose‐ranging study (Explore‐Xa). European Heart Journal 2013;34(20):1498‐505. [DOI: 10.1093/eurheartj/eht/039] - DOI - PMC - PubMed
J‐ROCKET AF 2012 {published and unpublished data}
OPAL‐1 2010 {published and unpublished data}
-
- Turpie AGGF, Lip GYH, Minematsu K, Goto S, Renfurm RW, Wong KSL. Safety and tolerability of YM150 in subjects with non‐valvular atrial fibrillation: a phase II study. European Heart Journal 2010;31 Suppl 1:173.
OPAL‐2 2011 {published data only}
-
- Lip GYH, Halperin JL, Petersen P, Rodger GM, Renfurm RW. Safety and tolerability of the oral factor Xa inhibitor YM150 vs warfarin in 1297 patients with non‐valvular atrial fibrillation: a dose confirmation study (OPAL‐2). Journal of Thrombosis and Haemostatis 2011;9 Suppl 2:748. - PubMed
ROCKET AF 2011 {published data only (unpublished sought but not used)}
-
- Patel MR, Mahaffey KW, Garg Y, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New England Journal of Medicine 2011;365:883‐91. - PubMed
References to studies excluded from this review
BAY59‐7939 Japan 2006 {unpublished data only}
-
- BAY59‐7936. ClinicalTrials.gov.
Camm 2011 {published data only}
-
- Camm AJ, Bounameaux H. Edoxaban: a new oral direct factor Xa inhibitor. Drugs 2011;71(12):1503‐26. - PubMed
Connolly 2011 {published data only}
-
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. New England Journal of Medicine 2011;364(9):806‐17. - PubMed
Harenberg 2010 {published data only}
-
- Harenberg J. Idraparinux and idrabioparinux. Expert Review of Clinical Pharmacology 2010;3(1):9‐16. - PubMed
Lopes 2010 {published data only}
-
- Lopes RD, Alexander JH, Al‐Khatib SM, the ARISTOTLE investigators. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. American Heart Journal 2010;159(3):331‐9. - PubMed
Partida 2011 {published data only}
-
- Partida RA, Guigliano RP. Edoxaban: pharmacological principles, preclinical and early‐phase clinical testing. Future Cardiology 2011;7(4):459‐70. - PubMed
ROCKET investigators 2010 {published data only}
-
- ROCKET AF Study Investigators. Rivaroxaban once‐daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: rationale and design of the ROCKET AF study. American Heart Journal 2010;159(3):340‐7. - PubMed
Study‐11866 2007 {unpublished data only}
-
- Study synopsis.
Additional references
ACC/AHA/HRS 2014
-
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, et al. Guidelines for the management of patients with atrial fibrillation. Circulation 2014; Vol. 129. [DOI: 10.1161/CIR.000000000000000041] - DOI
Almutairi 2016
-
- Almutairi A, Zhou L, Lee JK, Marion S, Martin JR, Lo‐Ciganic W. Comparative effectiveness and safety of novel oral anticoagulants (NOACS) versus vitamin K antagonists (VKA) in atrial fibrillation: a meta‐analysis of observational studies. Value Health 2016;3(19):A41.
Biondi‐Zoccai 2013
-
- Biondi‐Zoccai G, Malavasi V, D’Ascenzo F, Abbate A, Agostoni P, Lotrionte M, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair‐wise and warfarin‐controlled network meta‐analyses. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2013;5(1):40‐54. - PMC - PubMed
Boulanger 2006
-
- Boulanger L, Kim J, Friedman M, Hauch O, Foster T, Menzin J. Patterns of use of antithrombotic therapy and quality of anticoagulation monitoring among patients with non‐valvular atrial fibrillation in clinical practice. International Journal of Clinical Practice 2006;60:258‐64. - PubMed
Caldeira 2015
-
- Caldeira D, Rodrigues FB, Barra M, Santos AT, Abreu D, Goncalves N. Non‐vitamin K antagonist oral anticoagulants and major bleeding‐related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta‐analysis. Heart 2015;15(101):1204‐11. - PubMed
Cameron 2014
-
- Cameron C, Coyle D, Richter T, Kelly S, Gauthier K, Steiner S, et al. Systematic review and network meta‐analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open 2014;4(6):e004301. [DOI: 10.1136/bmjopen-2013-004301] - DOI - PMC - PubMed
Capodanno 2013
CHADS2
-
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285(22):2864‐70. - PubMed
Cochrane Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Connolly 2007
-
- Connolly SJ, Eikelboom J, O'Donnell M, Pogue J, Yusuf S. Challenges of establishing new antithrombotic therapies in atrial fibrillation. Circulation 2007;116:449‐55. - PubMed
Connolly 2008
-
- Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi G, the ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of International Normalized Ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118:2029‐37. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. Meta‐analysis in Context. London: BMJ Books, 2001:285‐312.
Dogliotti 2013
EHRA 2013
-
- Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association practical guide on the use of new oral anticoagulants in patients with non‐valvular atrial fibrillation. Europace 2013;15:625‐51. - PubMed
Eikelboom 2010
-
- Eikelboom JW, Weitz JI. New anticoagulants. Circulation 2010;121:1523‐32. - PubMed
ESC 2016
-
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal 2016;37:2893‐962. - PubMed
Fu 2014
Garg 2016
-
- Garg J, Chaudhary R, Krishnamoorthy P, Palaniswamy C, Shah N, Bozorgnia B, et al. Safety and efficacy of oral factor‐Xa inhibitors versus vitamin K antagonist in patients with non‐valvular atrial fibrillation. International Journal of Cardiology 2016;218:235‐9. - PubMed
Go 2001
-
- Go AS, Hylek EM, Philips KA, Henault, LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370‐5. - PubMed
Gomez‐Outes 2015
-
- Gomez‐Outes A, Suarez‐Gea ML, Terleira‐Fernandez AI, Vargas‐Castrillon E, Calvo‐Rojas G. Direct oral anticoagulants versus warfarin for stroke prevention in atrial fibrillation: a subgroup meta‐analysis in European patients. Clinical Therapeutics 2015;37(8 Suppl):e10.
Haim 2015
-
- Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non‐valvular atrial fibrillation. Journal of the American Heart Association 2015;4:e001486. - PMC - PubMed
Hankey 2012
-
- Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurology 2012;11:315‐22. - PubMed
Hart 2007
-
- Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have non‐valvular atrial fibrillation. Annals of Internal Medicine 2007;146:857‐67. - PubMed
Heeringa 2006
-
- Heeringa J, Kuip DA, Hofman A, Kors JA, Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. European Heart Journal 2006;27:949‐53. - PubMed
Henriksson 2012
-
- Henriksson KM, Farahmand B, Asberg S, Edvardsson N, Terent A. Comparison of cardiovascular risk factors and survival in patients with ischemic or hemorrhagic stroke. International Journal of Stroke 2012;7:276‐81. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hylek 2007
-
- Hylek EM, Evans‐Molina C, Shea C, Henault LE, Regan S. Major haemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689‐96. - PubMed
Kirchhof 2007
-
- Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener H‐C, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork (AFNET) and the European Heart Rhythm Association (EHRA). European Heart Journal 2007;28:2803‐17. - PubMed
Krijthe 2013
Kwong 2013
Lega 2013
Lin 2015
-
- Lin L, Lim WS, Zhou HJ, Khoo AL, Tan KT, Chew AP, et al. Clinical and safety outcomes of oral antithrombotics for stroke prevention in atrial fibrillation: a systematic review and network meta‐analysis. Journal of the American Medical Directors Association 2015;16(12):1103.e1‐19. - PubMed
Lip 2016
-
- Lip GYH, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, et al. Relative efficacy and safety of non‐Vitamin K oral anticoagulants for non‐valvular atrial fibrillation: network meta‐analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Internaitonal Journal of Cardiology 2016;204:88‐94. - PubMed
Liu 2016
-
- Liu L, Weber B, Oesterle A, Nayak HM, Upadhyay GA. A meta‐analysis of the efficacy, effectiveness, and safety of novel oral anticoagulants compared with vitamin K antagonists for stroke prevention in atrial fibrillation. Heart Rhythm 2016;1:S599.
Lloyd‐Jones 2004
-
- Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation 2004;110:1042‐6. - PubMed
Marini 2005
-
- Marini C, Santis F, Sacco S, Russo T, Olivieri L, Totaro R, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Results from a population study. Stroke 2005;36:1115‐9. - PubMed
Mitchell 2013
-
- Mitchell SA, Simon TA, Raza S, Jakouloff D, Orme ME, Lockhart I, et al. The efficacy and safety of oral anticoagulants in warfarin‐suitable patients with nonvalvular atrial fibrillation: systematic review and meta‐analysis. Clinical and Applied Thrombosis/Hemostasis 2013;19(6):619‐31. [DOI: 10.1177/1076029613486539] - DOI - PubMed
Miyasaki 2006
-
- Miyasaki Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projection for future prevalence. Circulation 2006;114:119‐25. - PubMed
Morimoto 2015
-
- Morimoto T, Crawford B, Wada K, Ueda S. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta‐analysis with the adjustment for the possible bias from open label studies. Journal of Cardiology 2015;66(6):466‐74. - PubMed
Mousa 2010
-
- Mousa SA. Oral direct factor Xa inhibitors, with special emphasis on rivaroxaban. Anticoagulants, Antiplatelets, and Thrombolytics. Springer Science+Business Media, 2010:181‐201. - PubMed
Pisters 2010
-
- Pisters R, Lane DA, Nieuwlaat R, Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010;138(5):1093‐100. - PubMed
Providencia 2014
-
- Providência R, Grove EL, Husted S, Barra S, Boveda S, Morais J. A meta‐analysis of phase III randomized controlled trials with novel oral anticoagulants in atrial fibrillation: comparisons between direct thrombin inhibitors vs. factor Xa inhibitors and different dosing regimens. Thrombosis Research 2014;134(6):1253‐64. [DOI: 10.1016/j.thromres.2014.10.002] - DOI - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruff 2014
-
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;15(383):955‐62. [DOI: 10.1016/S0140-6736(13)62343-0] - DOI - PubMed
Sudlow 1997
Tahir 2013
Tawfik 2016
Watson 2009
-
- Watson T, Shantsila E, Lip GYH. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373:155‐66. - PubMed
Wattigney 2003
-
- Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalisation for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 2003;108:711‐6. - PubMed
Wolf 1991
-
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983‐8. - PubMed
Xiang 2016
-
- Xiang CL, Gong YZ, Zeng LJ, Wang R, Kea S, Chaudhary N, et al. Efficacy and safety or oral direct factor Xa inhibitors versus warfarin in patients with atrial fibrillation: a meta‐analysis of randomized controlled trials. Acta Cardiologica 2016;71(3):349‐57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous