Characterization of solution-phase drug-protein interactions by ultrafast affinity extraction
- PMID: 29510250
- PMCID: PMC6098730
- DOI: 10.1016/j.ymeth.2018.02.021
Characterization of solution-phase drug-protein interactions by ultrafast affinity extraction
Abstract
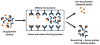
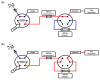
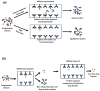
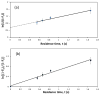
A number of tools based on high-performance affinity separations have been developed for studying drug-protein interactions. An example of one recent approach is ultrafast affinity extraction. This method has been employed to examine the free (or non-bound) fractions of drugs and other solutes in simple or complex samples that contain soluble binding agents. These free fractions have also been used to determine the binding constants and rate constants for the interactions of drugs with these soluble agents. This report describes the general principles of ultrafast affinity extraction and the experimental conditions under which it can be used to characterize such interactions. This method will be illustrated by utilizing data that have been obtained when using this approach to measure the binding and dissociation of various drugs with the serum transport proteins human serum albumin and alpha1-acid glycoprotein. A number of practical factors will be discussed that should be considered in the design and optimization of this approach for use with single-column or multi-column systems. Techniques will also be described for analyzing the resulting data for the determination of free fractions, rate constants and binding constants. In addition, the extension of this method to complex samples, such as clinical specimens, will be considered.
Keywords: Affinity microcolumn; Alpha(1)-acid glycoprotein; Drug-protein interactions; High performance affinity chromatography; Human serum albumin; Ultrafast affinity extraction.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures




References
-
- Vuignier K, Schappler J, Veuthey JL, Carrupt PA, Martel S. Drug-protein binding: a critical review of analytical tools. Anal Bioanal Chem. 2010;398:53–66. - PubMed
-
- Li Z, Beeram SR, Bi C, Suresh D, Zheng X, Hage DS. High-performance affinity chromatography: applications in drug-protein binding studies and personalized medicine. Adv Protein Chem Struct Biol. 2016;102:1–39. - PubMed
-
- Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99:1107–1122. - PubMed
-
- Wainer IW. The impact of new liquid chromatography chiral stationary phase technology on the study of stereoselective pharmacokinetics. Trends Anal Chem. 1993;12:153–158.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

