Restrictive Versus Massive Fluid Resuscitation Strategy (REFILL study), influence on blood loss and hemostatic parameters in obstetric hemorrhage: study protocol for a randomized controlled trial
- PMID: 29510717
- PMCID: PMC5838856
- DOI: 10.1186/s13063-018-2512-z
Restrictive Versus Massive Fluid Resuscitation Strategy (REFILL study), influence on blood loss and hemostatic parameters in obstetric hemorrhage: study protocol for a randomized controlled trial
Abstract
Background: Postpartum hemorrhage (PPH) is associated with maternal morbidity and mortality and has an increasing incidence in high-resource countries, despite dissemination of guidelines, introduction of skills training, and correction for risk factors. Current guidelines advise the administration, as fluid resuscitation, of almost twice the amount of blood lost. This advice is not evidence-based and could potentially harm patients.
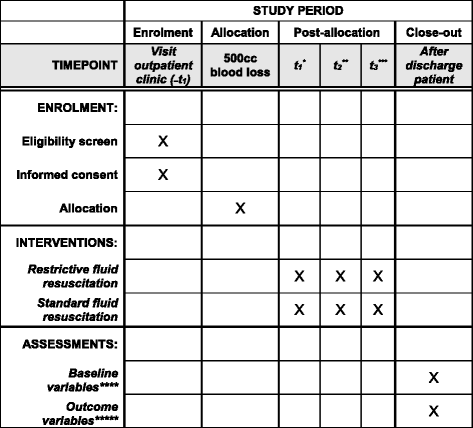
Methods: All women attending the outpatient clinic who are eligible will be informed of the study; oral and written informed consent will be obtained. Where there is more than 500 ml blood loss and ongoing bleeding, patients will be randomized to care as usual, fluid resuscitation with 1.5-2 times the amount of blood loss or fluid resuscitation with 0.75-1.0 times the blood loss. Blood loss will be assessed by weighing all draping. A blood sample, for determining hemoglobin concentration, hematocrit, thrombocyte concentration, and conventional coagulation parameters will be taken at the start of the study, after 60 min, and 12-18 h after delivery. In a subgroup of women, additional thromboelastometric parameters will be obtained.
Discussion: Our hypothesis is that massive fluid administration might lead to a progression of bleeding due to secondary coagulation disorders. In non-pregnant individuals with massive blood loss, restrictive fluid management has been shown to prevent a progression to dilution coagulopathy. These data, however, cannot be extrapolated to women in labor. Our objective is to compare both resuscitation protocols in women with early, mild PPH (blood loss 500-750 ml) and ongoing bleeding, taking as primary outcome measure the progression to severe PPH (blood loss > 1000 ml).
Trial registration: Netherlands Trial Register, NTR 3789 . Registered on 11 January 2013.
Keywords: Hemostatic parameters; Liberal fluid resuscitation; Postpartum hemorrhage; Randomized controlled trial; Restrictive fluid resuscitation.
Conflict of interest statement
Ethics approval and consent to participate
This study is approved by the Medical Ethics Committee Maastricht University Hospital (approval number, NL4294206813). This trial is registered in the Netherlands Trial Register NTR 3789 (date of registration, 11 January 2013). We have obtained informed consent from all participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Restrictive versus liberal fluid resuscitation strategy, influence on blood loss and hemostatic parameters in mild obstetric hemorrhage: An open-label randomized controlled trial. (REFILL study).PLoS One. 2021 Jun 25;16(6):e0253765. doi: 10.1371/journal.pone.0253765. eCollection 2021. PLoS One. 2021. PMID: 34170943 Free PMC article. Clinical Trial.
-
Restrictive versus liberal fluid administration strategy (REFILL study) in postpartum hemorrhage and its effects on thromboelastometry (ROTEM®) values: a randomized, controlled trial.J Int Med Res. 2023 Aug;51(8):3000605231171007. doi: 10.1177/03000605231171007. J Int Med Res. 2023. PMID: 37535070 Free PMC article. Clinical Trial.
-
Association between fluid management and dilutional coagulopathy in severe postpartum haemorrhage: a nationwide retrospective cohort study.BMC Pregnancy Childbirth. 2018 Oct 11;18(1):398. doi: 10.1186/s12884-018-2021-9. BMC Pregnancy Childbirth. 2018. PMID: 30305108 Free PMC article.
-
Massive obstetric hemorrhage: Current approach to management.Med Intensiva. 2016 Jun-Jul;40(5):298-310. doi: 10.1016/j.medin.2016.02.010. Epub 2016 May 13. Med Intensiva. 2016. PMID: 27184441 Review. English, Spanish.
-
Obstetric hemorrhage and coagulation: an update. Thromboelastography, thromboelastometry, and conventional coagulation tests in the diagnosis and prediction of postpartum hemorrhage.Obstet Gynecol Surv. 2012 Jul;67(7):426-35. doi: 10.1097/OGX.0b013e3182605861. Obstet Gynecol Surv. 2012. PMID: 22926249 Review.
Cited by
-
In Vivo Effects of Balanced Crystalloid or Gelatine Infusions on Functional Parameters of Coagulation and Fibrinolysis: A Prospective Randomized Crossover Study.J Pers Med. 2022 May 31;12(6):909. doi: 10.3390/jpm12060909. J Pers Med. 2022. PMID: 35743694 Free PMC article.
-
Fluid resuscitation in trauma: what are the best strategies and fluids?Int J Emerg Med. 2019 Dec 4;12(1):38. doi: 10.1186/s12245-019-0253-8. Int J Emerg Med. 2019. PMID: 31801458 Free PMC article. Review.
-
Effect of early restrictive fluid resuscitation on inflammatory and immune factors in patients with severe pelvic fracture.Chin J Traumatol. 2019 Dec;22(6):311-315. doi: 10.1016/j.cjtee.2019.07.008. Epub 2019 Sep 20. Chin J Traumatol. 2019. PMID: 31685356 Free PMC article.
-
Restrictive versus liberal fluid resuscitation strategy, influence on blood loss and hemostatic parameters in mild obstetric hemorrhage: An open-label randomized controlled trial. (REFILL study).PLoS One. 2021 Jun 25;16(6):e0253765. doi: 10.1371/journal.pone.0253765. eCollection 2021. PLoS One. 2021. PMID: 34170943 Free PMC article. Clinical Trial.
References
-
- World Health Organization . WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization; 2012. - PubMed
-
- Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: A nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115(7):842–850. doi: 10.1111/j.1471-0528.2008.01713.x. - DOI - PubMed
-
- Briley A, Seed PT, Tydeman G, Ballard H, Waterstone M, Sandall J, Poston L, Tribe RM, Bewley S. Reporting errors, incidence and risk factors for postpartum haemorrhage and progression to severe PPH: A prospective observational study. BJOG. 2014;121(7):876–888. doi: 10.1111/1471-0528.12588. - DOI - PMC - PubMed
-
- Netherlands Perinatal Registry. https://www.perined.nl/. Accessed 4 Jan 2013.
-
- Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, Joseph KS, Lewis G, Liston RM, Roberts CL, et al. Trends in postpartum hemorrhage in high resource countries: A review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

