Heme oxygenase-1 promoter region (GT)n polymorphism associates with increased neuroimmune activation and risk for encephalitis in HIV infection
- PMID: 29510721
- PMCID: PMC5838989
- DOI: 10.1186/s12974-018-1102-z
Heme oxygenase-1 promoter region (GT)n polymorphism associates with increased neuroimmune activation and risk for encephalitis in HIV infection
Abstract
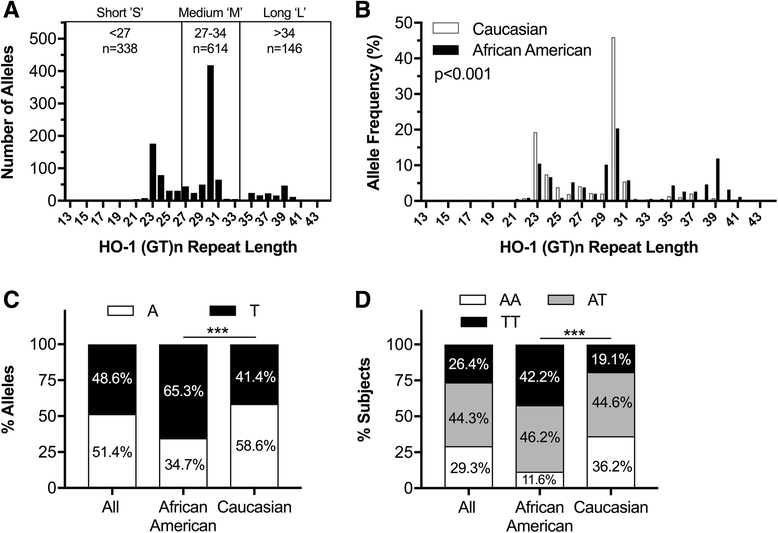
Background: Heme oxygenase-1 (HO-1) is a critical cytoprotective enzyme that limits oxidative stress, inflammation, and cellular injury within the central nervous system (CNS) and other tissues. We previously demonstrated that HO-1 protein expression is decreased within the brains of HIV+ subjects and that this HO-1 reduction correlates with CNS immune activation and neurocognitive dysfunction. To define a potential CNS protective role for HO-1 against HIV, we analyzed a well-characterized HIV autopsy cohort for two common HO-1 promoter region polymorphisms that are implicated in regulating HO-1 promoter transcriptional activity, a (GT)n dinucleotide repeat polymorphism and a single nucleotide polymorphism (A(-413)T). Shorter HO-1 (GT)n repeats and the 'A' SNP allele associate with higher HO-1 promoter activity.
Methods: Brain dorsolateral prefrontal cortex tissue samples from an autopsy cohort of HIV-, HIV+, and HIV encephalitis (HIVE) subjects (n = 554) were analyzed as follows: HO-1 (GT)n polymorphism allele lengths were determined by PCR and capillary electrophoresis, A(-413)T SNP alleles were determined by PCR with allele specific probes, and RNA expression of selected neuroimmune markers was analyzed by quantitative PCR.
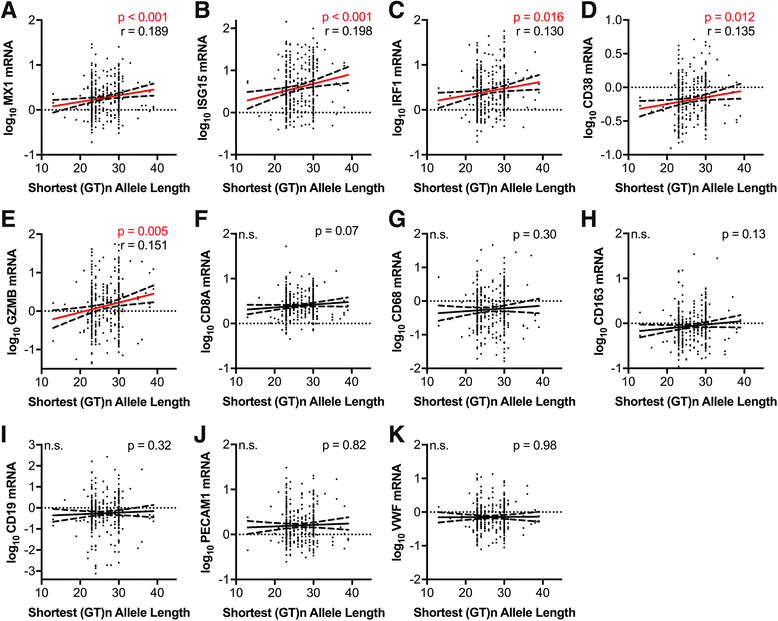
Results: HIV+ subjects with shorter HO-1 (GT)n alleles had a significantly lower risk of HIVE; however, shorter HO-1 (GT)n alleles did not correlate with CNS or peripheral viral loads. In HIV+ subjects without HIVE, shorter HO-1 (GT)n alleles associated significantly with lower expression of brain type I interferon response markers (MX1, ISG15, and IRF1) and T-lymphocyte activation markers (CD38 and GZMB). No significant correlations were found between the HO-1 (GT)n repeat length and brain expression of macrophage markers (CD163, CD68), endothelial markers (PECAM1, VWF), the T-lymphocyte marker CD8A, or the B-lymphocyte maker CD19. Finally, we found no significant associations between the A(-413)T SNP and HIVE diagnosis, HIV viral loads, or any neuroimmune markers.
Conclusion: Our data suggest that an individual's HO-1 promoter region (GT)n polymorphism allele repeat length exerts unique modifying risk effects on HIV-induced CNS neuroinflammation and associated neuropathogenesis. Shorter HO-1 (GT)n alleles increase HO-1 promoter activity, which could provide neuroprotection through decreased neuroimmune activation. Therapeutic strategies that induce HO-1 expression could decrease HIV-associated CNS neuroinflammation and decrease the risk for development of HIV neurological disease.
Keywords: HAND; HIV-associated neurocognitive disorders; HMOX1; HO-1; Heme oxygenase-1; Immune activation; Interferon; Interferon-stimulated genes; Neuroinflammation.
Conflict of interest statement
Ethics approval and consent to participate
All NNTC studies were conducted in accordance with human subject protection protocols at participating institutions. Written informed consent was obtained for subjects at four collection sites in the USA. The following offices maintained the Institutional Review Boards that provided oversight for the protection of human subjects: (1) The University of Texas Medical Branch Office of Research Subject Protections; (2) Mount Sinai Medical Center Program for the Protection of Human Subjects; (3) University of California, San Diego Human Research Protections Program; and (4) University of California, Los Angeles Office of the Human Research Protection Program.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Heme oxygenase-1 promoter (GT) n polymorphism associates with HIV neurocognitive impairment.Neurol Neuroimmunol Neuroinflamm. 2020 Apr 10;7(3):e710. doi: 10.1212/NXI.0000000000000710. Print 2020 May. Neurol Neuroimmunol Neuroinflamm. 2020. PMID: 32277015 Free PMC article.
-
GT-repeat polymorphism in the heme oxygenase-1 gene promoter and the risk of carotid atherosclerosis related to arsenic exposure.J Biomed Sci. 2010 Aug 26;17(1):70. doi: 10.1186/1423-0127-17-70. J Biomed Sci. 2010. PMID: 20796278 Free PMC article.
-
GT-repeat polymorphism in the heme oxygenase-1 gene promoter is associated with cardiovascular mortality risk in an arsenic-exposed population in northeastern Taiwan.Toxicol Appl Pharmacol. 2010 Nov 1;248(3):226-33. doi: 10.1016/j.taap.2010.08.005. Epub 2010 Aug 12. Toxicol Appl Pharmacol. 2010. PMID: 20708634 Clinical Trial.
-
Heme oxygenase-1 gene promoter polymorphisms are associated with coronary heart disease and restenosis after percutaneous coronary intervention: a meta-analysis.Oncotarget. 2016 Dec 13;7(50):83437-83450. doi: 10.18632/oncotarget.13118. Oncotarget. 2016. PMID: 27825138 Free PMC article. Review.
-
HMOX1 genetic polymorphisms and outcomes in infectious disease: A systematic review.PLoS One. 2022 May 12;17(5):e0267399. doi: 10.1371/journal.pone.0267399. eCollection 2022. PLoS One. 2022. PMID: 35551540 Free PMC article.
Cited by
-
Developments in Neuroprotection for HIV-Associated Neurocognitive Disorders (HAND).Curr HIV/AIDS Rep. 2022 Oct;19(5):344-357. doi: 10.1007/s11904-022-00612-2. Epub 2022 Jul 22. Curr HIV/AIDS Rep. 2022. PMID: 35867211 Free PMC article. Review.
-
Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated With Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma.J Infect Dis. 2019 Nov 6;220(12):1885-1891. doi: 10.1093/infdis/jiz030. J Infect Dis. 2019. PMID: 30668739 Free PMC article.
-
Heme oxygenase-1 modulation: A potential therapeutic target for COVID-19 and associated complications.Free Radic Biol Med. 2020 Dec;161:263-271. doi: 10.1016/j.freeradbiomed.2020.10.016. Epub 2020 Oct 19. Free Radic Biol Med. 2020. PMID: 33091573 Free PMC article. Review.
-
The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress.Redox Biol. 2021 Nov;47:102170. doi: 10.1016/j.redox.2021.102170. Epub 2021 Oct 20. Redox Biol. 2021. PMID: 34688156 Free PMC article. Review.
-
Comprehensive analyses identify potential biomarkers for encephalitis in HIV infection.Sci Rep. 2023 Oct 27;13(1):18418. doi: 10.1038/s41598-023-45922-6. Sci Rep. 2023. PMID: 37891420 Free PMC article.
References
-
- Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, Jordan-Sciutto KL, Kolson DL. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol. 2011;187:5015–5025. doi: 10.4049/jimmunol.1101868. - DOI - PMC - PubMed
-
- Gill AJ, Kovacsics CE, Vance PJ, Collman RG, Kolson DL. Induction of heme oxygenase-1 deficiency and associated glutamate-mediated neurotoxicity is a highly conserved HIV phenotype of chronic macrophage infection that is resistant to antiretroviral therapy. J Virol. 2015;89:10656–10667. doi: 10.1128/JVI.01495-15. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- U24 MH100931/MH/NIMH NIH HHS/United States
- T32 NS007180/NS/NINDS NIH HHS/United States
- F30MH102120/MH/NIMH NIH HHS/United States
- U24 MH100928/MH/NIMH NIH HHS/United States
- R01 MH104134/MH/NIMH NIH HHS/United States
- R01MH101017/MH/NIMH NIH HHS/United States
- R01 MH095671/MH/NIMH NIH HHS/United States
- R01 MH101017/MH/NIMH NIH HHS/United States
- U24 MH100930/MH/NIMH NIH HHS/United States
- R01 MH111389/MH/NIMH NIH HHS/United States
- T32NS007180/NS/NINDS NIH HHS/United States
- R01MH095671/MH/NIMH NIH HHS/United States
- F30 MH102120/MH/NIMH NIH HHS/United States
- U24 MH100929/MH/NIMH NIH HHS/United States
- R01MH104134/MH/NIMH NIH HHS/United States
- U24 MH100925/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

