MicroRNA-1468 promotes tumor progression by activating PPAR-γ-mediated AKT signaling in human hepatocellular carcinoma
- PMID: 29510736
- PMCID: PMC5839011
- DOI: 10.1186/s13046-018-0717-3
MicroRNA-1468 promotes tumor progression by activating PPAR-γ-mediated AKT signaling in human hepatocellular carcinoma
Retraction in
-
Retraction Note: MicroRNA-1468 promotes tumor progression by activating PPAR-γ-mediated AKT signaling in human hepatocellular carcinoma.J Exp Clin Cancer Res. 2022 Sep 13;41(1):272. doi: 10.1186/s13046-022-02487-y. J Exp Clin Cancer Res. 2022. PMID: 36096795 Free PMC article. No abstract available.
Abstract
Background: Accumulating evidence confirm that aberrant microRNAs (miRNAs) expression contributes to hepatocellular carcinoma (HCC) development and progression. Previous study reported that miR-1468 showed an up-regulated tendency and might be a potential prognostic biomarker in HCC samples derived from TCGA database. However, the role of miR-1468 and its underlying mechanisms involved in the growth and metastasis of HCC remain poorly investigated.
Methods: CCK-8, EdU, colony formation and flow cytometry were used to determine proliferation, cell cycle progression and apoptosis of HCC cells in vitro. The subcutaneous tumor model in nude mice was established to detect tumor growth of HCC in vivo. The direct binding of miR-1468 to 3'UTR of Cbp/p300 interacting transactivator with Glu/Asp rich carboxy-terminal domain 2 (CITED2) and Up-frameshift protein 1 (UPF1) was confirmed by luciferase reporter assay.
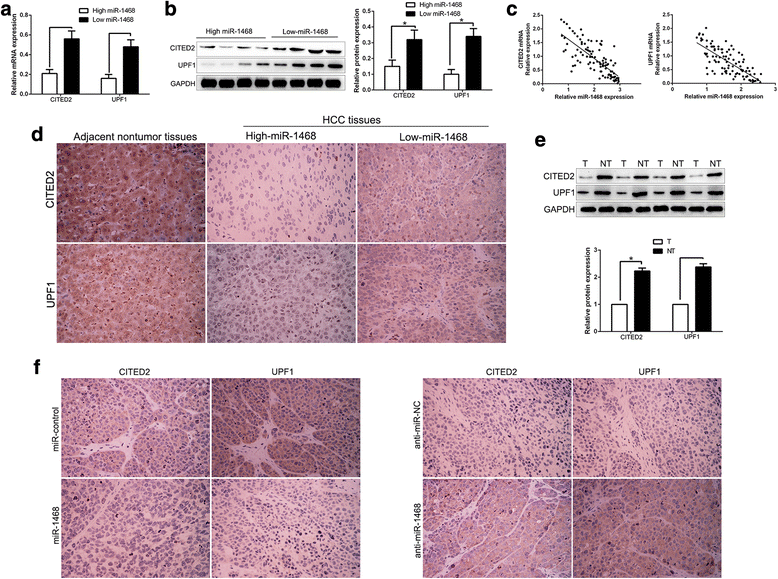
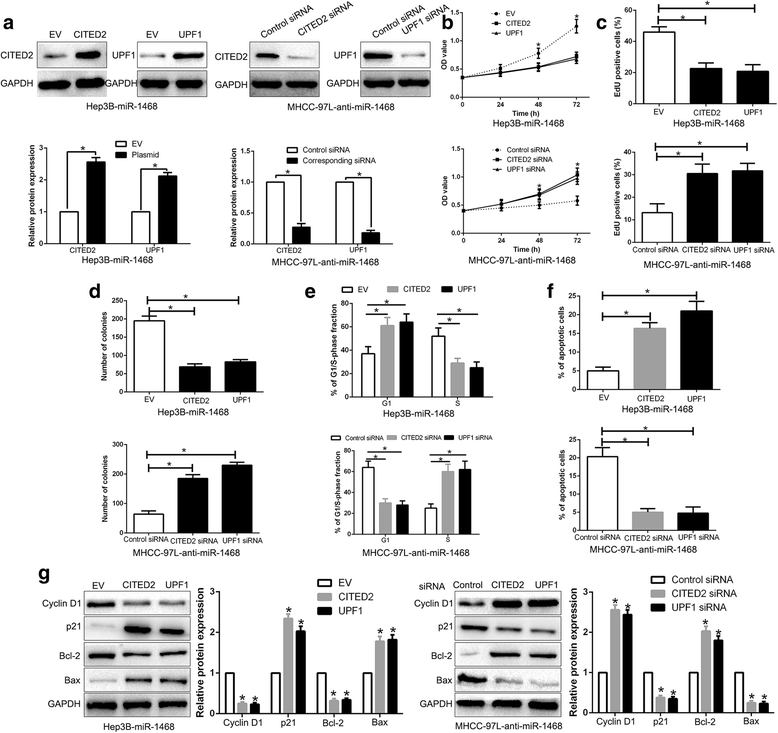
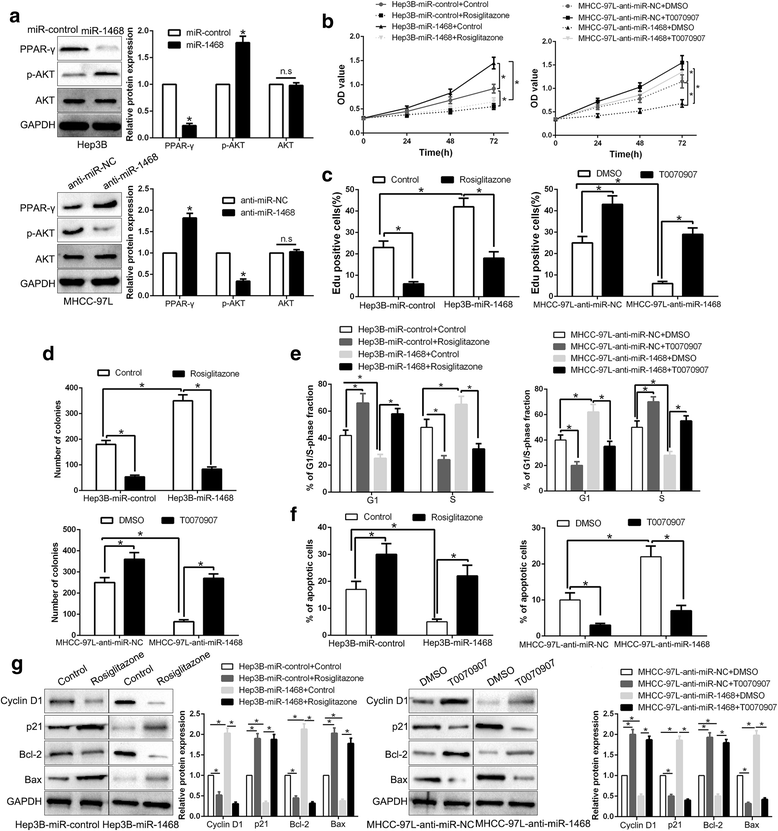
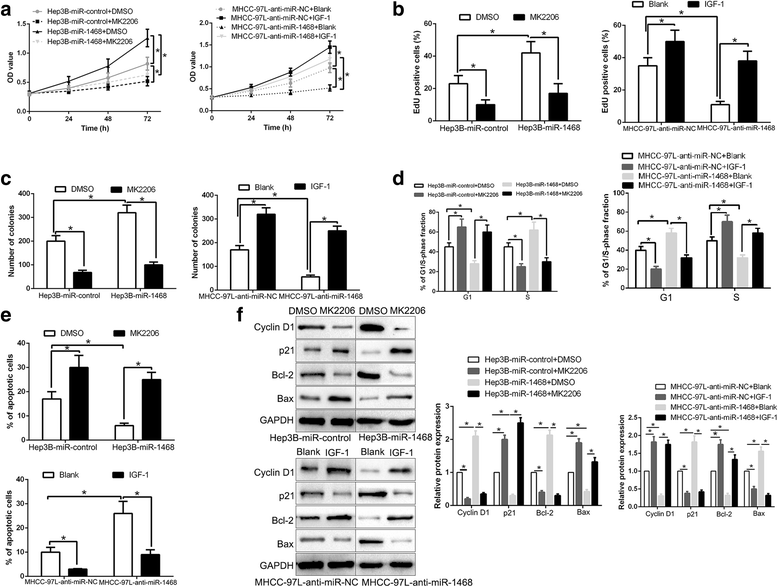
Results: Here, we demonstrated that miR-1468 expression was up-regulated in HCC tissues and cell lines. Clinical analysis revealed that increased miR-1468 level was significantly correlated with malignant prognostic features and shorter survival. Gain- and loss-of-function experiments indicated that miR-1468 promoted cell proliferation, colony formation, cell cycle progression and induced apoptosis of HCC cells in vitro and in vivo. Moreover, CITED2 and UPF1 were identified as direct downstream targets of miR-1468 in HCC cells, and mediated the functional effects of miR-1468 in HCC, resulting in peroxisome proliferator-activated receptor-γ (PPAR-γ)/AKT signaling activation. In clinical samples of HCC, miR-1468 inversely correlated with the levels of CITED2 and UPF1, which were confirmed to be down-regulated in HCC. Restoration of CITED2 or UPF1 expression at least partially abolished the biological effects of miR-1468 on HCC cells. Moreover, alteration of PPAR-γ or AKT phosphorylation could reverse the function of miR-1468 in HCC.
Conclusions: Taken together, this research supports the first evidence that miR-1468 plays an oncogenic role in HCC via activating PPAR-γ/AKT pathway by targeting CITED2 and UPF1, and represents a promising therapeutic strategy for HCC patients.
Keywords: CITED2; Hepatocellular carcinoma; PPAR-γ; Tumor growth; UPF2; miR-1468.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University and with the 1964 Helsinki declaration and its later amendments. ALL written informed consent to participate in the study was obtained from HCC patients for samples to be collected from them.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

