Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in metastatic melanoma: 5-year follow up and additional analyses
- PMID: 29510745
- PMCID: PMC5840808
- DOI: 10.1186/s40425-018-0330-1
Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in metastatic melanoma: 5-year follow up and additional analyses
Abstract
Background: Despite improved survival following checkpoint inhibitors, there is still a potential role for anti-cancer therapeutic vaccines. Because of biological heterogeneity and neoantigens resulting from each patient's mutanome, autologous tumor may be the best source of tumor-associated antigens (TAA) for vaccines. Ex vivo loading of autologous dendritic cells with TAA may be associated with superior clinical outcome compared to injecting irradiated autologous tumor cells. We conducted a randomized phase II trial to compare autologous tumor cell vaccines (TCV) and autologous dendritic cell vaccines (DCV) loaded with autologous TAA.
Methods: Short-term autologous tumor cell lines were established from metastatic tumor. Vaccines were admixed with 500 micrograms of GM-CSF and injected weekly for 3 weeks, then at weeks 8, 12,16, 20, and 24. The primary endpoint was overall survival. Secondary objectives were identification of adverse events, and results of delayed type hypersensitivity (DTH) reactions to intradermal tumor cell injections.
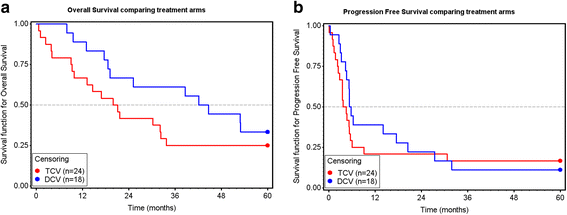
Results: Forty-two patients were randomized. All were followed from randomization until death or for five years; none were lost to follow-up. DCV was associated with longer survival: median 43.4 versus 20.5 months (95% CI, 18.6 to > 60 versus 9.3 to 32.3 months) and a 70% reduction in the risk of death (hazard ratio = 0.304, p = 0.0053, 95% CI, 0.131 to 0.702). Tumor DTH reactions were neither prognostic nor predictive. The most common treatment-related adverse events were mild to moderate local injection site reactions and flu-like symptoms; but grade 2 treatment-related adverse events were more frequent with TCV. Serum marker analyses at week-0 and week-4 showed that serum markers were similar at baseline in each arm, but differed after vaccination.
Conclusions: This is the only human clinical trial comparing DCV and TCV as platforms for autologous TAA presentation. DCV was associated with minimal toxicity and long-term survival in patients with metastatic melanoma. DTH to autologous tumor cells was neither prognostic for survival nor predictive of benefit for either vaccine.
Trial registration: Clinical trials.gov NCT00948480 retrospectively registered 28 July 2009.
Keywords: Autologous tumor cell lines; Dendritic-cell vaccines; Metastatic melanoma; Patient-specific vaccines; Tumor-cell vaccines.
Conflict of interest statement
Authors’ information
ROD is a former president of the Society for Immunotherapy of Cancer (2000–2002, then called the International Society for the Biotherapy of Cancer) after serving two previous terms on its Board of Directors. For more than a decade he was Chairman of the Cancer Biotherapy Research Group. From 1989 to 2011 he was Medical Director of the Hoag Cancer Center, where this trial was conducted, and Scientific Director for the Hoag Cell Biology Laboratory, where the investigational products used in this trial were manufactured.
Ethics approval and consent to participate
The MACVAC clinical trial was conducted per the Declaration of Helsinki after approval by the Western Institutional Review Board (Seattle, WA), and in accord with assurances filed with and approved by the Department of Health and Human Services. All patients gave written informed consent prior to randomization. In addition to Institutional Review Board oversight, conduct of the trial was monitored by an external consultant, and Hoag Hospital’s institutional clinical research oversight committee. The protocol and manufacturing procedures were reviewed by the US Food and Drug Administration in association with BB-IND 5838 for the tumor cell vaccine, 8554 for the dendritic cell vaccine. The trial was registered on ClinicalTrials.gov (NCT00436930) on 28 July 2009.
Consent for publication
Not applicable
Competing interests
ROD is an employ of AIVITA Biomedical, Inc. ANC is an employee of TCR2. GIN is an employ of AVITIA Biomedical, Inc. ROD, ANC, and GIN are former employees of Caladrius Biosciences, Inc. and retain stock in that company. None of the other authors declared any conflicts as related to this trial.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
