Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies
- PMID: 29510782
- PMCID: PMC5840921
- DOI: 10.2807/1560-7917.ES.2018.23.9.18-00086
Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies
Abstract
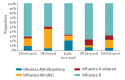
Between September 2017 and February 2018, influenza A(H1N1)pdm09, A(H3N2) and B viruses (mainly B/Yamagata, not included in 2017/18 trivalent vaccines) co-circulated in Europe. Interim results from five European studies indicate that, in all age groups, 2017/18 influenza vaccine effectiveness was 25 to 52% against any influenza, 55 to 68% against influenza A(H1N1)pdm09, -42 to 7% against influenza A(H3N2) and 36 to 54% against influenza B. 2017/18 influenza vaccine should be promoted where influenza still circulates.
Keywords: Europe; case control study; influenza; influenza vaccination; influenza vaccine effectiveness; multicentre study.
Conflict of interest statement
Figures
References
-
- European Centre for Disease Prevention and Control (ECDC). Seasonal influenza vaccination and antiviral use in Europe. Overview of vaccination recommendations and coverage rates in the EU Member States for the 2013-14 and 2014-15 influenza seasons. VENICE technical report [Internet]. Stockholm: ECDC; 2016. Available from: http://venice.cineca.org/Seasonal-influenza-vaccination-antiviral-use-eu...
-
- Hakin B, Cosford P, Harvey F. The flu immunisation programme 2013/14– extension to children. London: Department of Health; 2013. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil...
-
- World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2017-2018 northern hemisphere influenza season. Geneva: WHO; 2017. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2017_18_nort...
-
- European Centre for Disease Prevention and Control (ECDC), World Health Organization Regional Office for Europe (WHO/Europe). Flu News Europe. Summary week 7/2018 (12-18 February 2018). Stockholm: ECDC, Copenhagen: WHO/Europe; 2018. Available from: http://flunewseurope.org/Archives
-
- I-MOVE+. Protocol for hospital-based test negative case control studies to measure seasonal influenza vaccine effectiveness against influenza laboratory confirmed SARI hospitalisation among the elderly across the European Union and European Economic Area Member States. Paris: EpiConcept; 2016. Available from: https://drive.google.com/file/d/0B54XpZN4SY65OGRSTHlvOVZTMFE/view
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical