A chemically stable fluorescent marker of the ureter
- PMID: 29510880
- PMCID: PMC6437767
- DOI: 10.1016/j.bmcl.2018.02.040
A chemically stable fluorescent marker of the ureter
Abstract
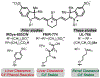
Surgical methods guided by exogenous fluorescent markers have the potential to define tissue types in real time. Small molecule dyes with efficient and selective renal clearance could enable visualization of the ureter during surgical procedures involving the abdomen and pelvis. These studies report the design and synthesis of a water soluble, net neutral C4'-O-alkyl heptamethine cyanine, Ureter-Label (UL)-766, with excellent properties for ureter visualization. This compound is accessed through a concise synthetic sequence involving an N- to O-transposition reaction that provides other inaccessible C4'-O-alkyl heptamethine cyanines. Unlike molecules containing a C4'-O-aryl substituent, which have also been used for ureter visualization, UL-766 is not reactive towards glutathione and the cellular proteome. In addition, rat models of abdominal surgery reveal that UL-766 undergoes efficient and nearly exclusive renal clearance in vivo. In total, this molecule represents a promising candidate for visualizing the ureter during a variety of surgical interventions.
Keywords: Fluorescence-guided surgery; Fluorophore synthesis; Near-IR fluorescence.
Published by Elsevier Ltd.
Figures



References
-
- Perrin DP, Vasilyev NV, Novotny P, et al. Image guided surgical interventions. Curr Probl Surg 2009;46: 730–766. - PubMed
-
- Martinic I, Eliseeva SV, Petoud S. Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials. J Lumin 2017;189: 19–43.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

