MicroRNA duplication accelerates the recruitment of new targets during vertebrate evolution
- PMID: 29511046
- PMCID: PMC5959248
- DOI: 10.1261/rna.062752.117
MicroRNA duplication accelerates the recruitment of new targets during vertebrate evolution
Abstract
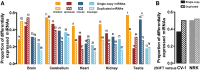
The repertoire of miRNAs has considerably expanded during metazoan evolution, and duplication is an important mechanism for generating new functional miRNAs. However, relatively little is known about the functional divergence between paralogous miRNAs and the possible coevolution between duplicated miRNAs and the genomic contexts. By systematically examining small RNA expression profiles across various human tissues and interrogating the publicly available miRNA:mRNA pairing chimeras, we found that changes in expression patterns and targeting preferences are widespread for duplicated miRNAs in vertebrates. Both the empirical interactions and target predictions suggest that evolutionarily conserved homo-seed duplicated miRNAs pair with significantly higher numbers of target sites compared to the single-copy miRNAs. Our birth-and-death evolutionary analysis revealed that the new target sites of miRNAs experienced frequent gains and losses during function development. Our results suggest that a newly emerged target site has a higher probability to be functional and maintained by natural selection if it is paired to a seed shared by multiple paralogous miRNAs rather than being paired to a single-copy miRNA. We experimentally verified the divergence in target repression between two paralogous miRNAs by transfecting let-7a and let-7b mimics into kidney-derived cell lines of four mammalian species and measuring the resulting transcriptome alterations by extensive high-throughput sequencing. Our results also suggest that the gains and losses of let-7 target sites might be associated with the evolution of repressiveness of let-7 across mammalian species.
Keywords: birth-and-death; expression divergence; let-7 family; microRNA duplication; target repression; target site evolution.
© 2018 Luo et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures





References
-
- Albalat R, Cañestro C. 2016. Evolution by gene loss. Nat Rev Genet 17: 379–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
