Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases
- PMID: 29511093
- PMCID: PMC5840331
- DOI: 10.1042/BCJ20170714
Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases
Abstract
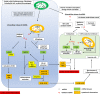
Mitochondria play a central role in multiple cellular functions, including energy production, calcium homeostasis, and cell death. Currently, growing evidence indicates the vital roles of mitochondria in triggering and maintaining inflammation. Chronic inflammation without microbial infection - termed sterile inflammation - is strongly involved in the development of heart failure. Sterile inflammation is triggered by the activation of pattern recognition receptors (PRRs) that sense endogenous ligands called damage-associated molecular patterns (DAMPs). Mitochondria release multiple DAMPs including mitochondrial DNA, peptides, and lipids, which induce inflammation via the stimulation of multiple PRRs. Among the mitochondrial DAMPs, mitochondrial DNA (mtDNA) is currently highlighted as the DAMP that mediates the activation of multiple PRRs, including Toll-like receptor 9, Nod-like receptors, and cyclic GMP-AMP synthetase/stimulator of interferon gene pathways. These PRR signalling pathways, in turn, lead to the activation of nuclear factor-κB and interferon regulatory factor, which enhances the transcriptional activity of inflammatory cytokines and interferons, and induces the recruitment of inflammatory cells. As the heart is an organ comprising abundant mitochondria for its ATP consumption (needed to maintain constant cyclic contraction and relaxation), the generation of massive amounts of mitochondrial radical oxygen species and mitochondrial DAMPs are predicted to occur and promote cardiac inflammation. Here, we will focus on the role of mtDNA in cardiac inflammation and review the mechanism and pathological significance of mtDNA-induced inflammatory responses in cardiac diseases.
Keywords: cardiovascular disease; inflammation; mtDNA.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

