Splenic leukocytes define the resolution of inflammation in heart failure
- PMID: 29511119
- PMCID: PMC6145858
- DOI: 10.1126/scisignal.aao1818
Splenic leukocytes define the resolution of inflammation in heart failure
Abstract
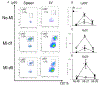
Inflammation promotes healing in myocardial infarction but, if unresolved, leads to heart failure. To define the inflammatory and resolving responses, we quantified leukocyte trafficking and specialized proresolving mediators (SPMs) in the infarcted left ventricle and spleen after myocardial infarction, with the goal of distinguishing inflammation from its resolution. Our data suggest that the spleen not only served as a leukocyte reservoir but also was the site where SPMs were actively generated after coronary ligation in mice. Before myocardial infarction, SPMs were more abundant in the spleen than in the left ventricle. At day 1 after coronary ligation, the spleen was depleted of leukocytes, a phenomenon that was associated with greater numbers of leukocytes in the infarcted left ventricle and increased generation of SPMs at the same site, particularly resolvins, maresin, lipoxins, and protectin. In addition, the infarcted left ventricle showed increased expression of genes encoding lipoxygenases and enhanced production of SPMs generated by these enzymes. We found that macrophages were necessary for SPM generation. The abundance of SPMs in the spleen before myocardial infarction and increased SPM concentrations in the infarcted left ventricle within 24 hours after myocardial infarction were temporally correlated with the resolution of inflammation. Thus, the acute inflammatory response coincided with the active resolving phase in post-myocardial infarction and suggests that further investigation into macrophage-derived SPMs in heart failure is warranted.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures






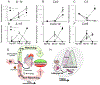
References
-
- Kain V, Prabhu SD, Halade GV, Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol 109, 444 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

