A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress
- PMID: 29511121
- PMCID: PMC5861378
- DOI: 10.1126/scisignal.aan6480
A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress
Abstract
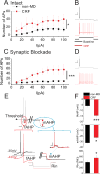
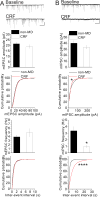
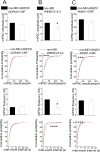
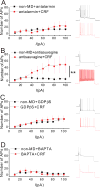
Centrally released corticotropin-releasing factor or hormone (extrahypothalamic CRF or CRH) in the brain is involved in the behavioral and emotional responses to stress. The lateral habenula (LHb) is an epithalamic brain region involved in value-based decision-making and stress evasion. Through its inhibition of dopamine-mediated reward circuitry, the increased activity of the LHb is associated with addiction, depression, schizophrenia, and behavioral disorders. We found that extrahypothalamic CRF neurotransmission increased neuronal excitability in the LHb. Through its receptor CRFR1 and subsequently protein kinase A (PKA), CRF application increased the intrinsic excitability of LHb neurons by affecting changes in small-conductance SK-type and large-conductance BK-type K+ channels. CRF also reduced inhibitory γ-aminobutyric acid-containing (GABAergic) synaptic transmission onto LHb neurons through endocannabinoid-mediated retrograde signaling. Maternal deprivation is a severe early-life stress that alters CRF neural circuitry and is likewise associated with abnormal mental health later in life. LHb neurons from pups deprived of maternal care exhibited increased intrinsic excitability, reduced GABAergic transmission, decreased abundance of SK2 channel protein, and increased activity of PKA, without any substantial changes in Crh or Crhr1 expression. Furthermore, maternal deprivation blunted the response of LHb neurons to subsequent, acute CRF exposure. Activating SK channels or inhibiting postsynaptic PKA activity prevented the effects of both CRF and maternal deprivation on LHb intrinsic excitability, thus identifying potential pharmacological targets to reverse central CRF circuit dysregulation in patients with associated disorders.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

