Low population serum microneutralization antibody titer against the predominating influenza A(H3N2) N121K virus during the severe influenza summer peak of Hong Kong in 2017
- PMID: 29511175
- PMCID: PMC5841213
- DOI: 10.1038/s41426-018-0041-1
Low population serum microneutralization antibody titer against the predominating influenza A(H3N2) N121K virus during the severe influenza summer peak of Hong Kong in 2017
Abstract
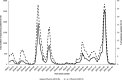
The 2017 Hong Kong influenza A(H3N2) summer season was unexpectedly severe. However, antigenic characterization of the 2017 circulating A(H3N2) viruses using ferret antisera did not show significant antigenic drift. We analyzed the hemagglutinin amino acid sequences of A(H3N2) virus circulating in Hong Kong in 2017, and found that viruses with hemagglutinin N121K substitution, which was rare before 2017, emerged rapidly and dominated in 2017 (52.4% of A[H3N2] virus in 2017 contains N121K substitution). Microneutralization assay using archived human sera collected from mid-2017 showed that the geometric mean microneutralization titer was 3.6-fold lower against a 2017 cell culture-grown circulating A(H3N2)-N121K virus (3391/2017 virus) than that against the cell culture-grown 2016-2017 A(H3N2) seasonal influenza vaccine-like vaccine virus (4801/2014 virus) (13.4 vs 41.8, P < 0.0001). Significantly fewer serum specimens had a microneutralization titer of 40 or above against 3391/2017 virus than that against 4801/2014 virus (26.4% vs 60.0%, P < 0.0001). Conversely, the geometric mean hemagglutination inhibition titer was slightly higher against 3391/2017 virus than that against the 4801/2014 virus (96.9 vs 55.4, P < 0.0001). Moreover, 59.1% of specimens had a significantly lower microneutralization antibody titer (≥4-fold) against 3391/2017 virus than that against 4801/2014 virus, but none for hemagglutination titer (P < 0.0001). Similar results of microneutralization and hemagglutination titers were observed for day 21-post-vaccination sera. Hence, the 2017 A(H3N2) summer peak in Hong Kong was associated with a low-microneutralization titer against the circulating virus. Our results support the use of microneutralization assay with human serum in assessing population susceptibility and antigenic changes of A(H3N2) virus. Novel and available immunization approach, such as topical imiquimod followed by intradermal vaccination, to broaden the neutralizing antibody response of influenza vaccine should be considered.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Antibodies Against Egg- and Cell-Grown Influenza A(H3N2) Viruses in Adults Hospitalized During the 2017-2018 Influenza Season.J Infect Dis. 2019 May 24;219(12):1904-1912. doi: 10.1093/infdis/jiz049. J Infect Dis. 2019. PMID: 30721982 Free PMC article.
-
Neutralizing Antibody Responses to Antigenically Drifted Influenza A(H3N2) Viruses among Children and Adolescents following 2014-2015 Inactivated and Live Attenuated Influenza Vaccination.Clin Vaccine Immunol. 2016 Oct 4;23(10):831-839. doi: 10.1128/CVI.00297-16. Print 2016 Oct. Clin Vaccine Immunol. 2016. PMID: 27558294 Free PMC article.
-
Lack of neutralizing antibodies against influenza A viruses in adults during the 2022/2023 winter season - a serological study using retrospective samples collected in Hong Kong.Int J Infect Dis. 2023 Oct;135:1-4. doi: 10.1016/j.ijid.2023.07.008. Epub 2023 Jul 20. Int J Infect Dis. 2023. PMID: 37481108
-
Improving the selection and development of influenza vaccine viruses - Report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18-20 November 2015.Vaccine. 2017 Feb 22;35(8):1104-1109. doi: 10.1016/j.vaccine.2017.01.018. Epub 2017 Jan 25. Vaccine. 2017. PMID: 28131392 Free PMC article. Review.
-
Targeting H3N2 influenza: advancements in treatment and vaccine strategies.Expert Rev Anti Infect Ther. 2025 Jan;23(1):5-18. doi: 10.1080/14787210.2024.2443920. Epub 2025 Jan 3. Expert Rev Anti Infect Ther. 2025. PMID: 39688174 Review.
Cited by
-
Recent H9N2 avian influenza virus lost hemagglutination activity due to a K141N substitution in hemagglutinin.J Virol. 2024 Apr 16;98(4):e0024824. doi: 10.1128/jvi.00248-24. Epub 2024 Mar 11. J Virol. 2024. PMID: 38466094 Free PMC article.
-
Intense interseasonal influenza outbreaks, Australia, 2018/19.Euro Surveill. 2019 Aug;24(33):1900421. doi: 10.2807/1560-7917.ES.2019.24.33.1900421. Euro Surveill. 2019. PMID: 31431210 Free PMC article.
-
Molecular epidemiology survey and characterization of human influenza A viruses circulating among Palestinians in East Jerusalem and the West Bank in 2015.PLoS One. 2019 Mar 8;14(3):e0213290. doi: 10.1371/journal.pone.0213290. eCollection 2019. PLoS One. 2019. PMID: 30849093 Free PMC article.
-
Co-circulation and persistence of multiple A/H3N2 influenza variants in China.Emerg Microbes Infect. 2019;8(1):1157-1167. doi: 10.1080/22221751.2019.1648183. Emerg Microbes Infect. 2019. PMID: 31373538 Free PMC article.
-
IgG3 subclass antibodies recognize antigenically drifted influenza viruses and SARS-CoV-2 variants through efficient bivalent binding.bioRxiv [Preprint]. 2022 Sep 28:2022.09.27.509738. doi: 10.1101/2022.09.27.509738. bioRxiv. 2022. Update in: Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2216521120. doi: 10.1073/pnas.2216521120. PMID: 36203556 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
