Group 2 innate lymphoid cells protect lung endothelial cells from pyroptosis in sepsis
- PMID: 29511181
- PMCID: PMC5840374
- DOI: 10.1038/s41419-018-0412-5
Group 2 innate lymphoid cells protect lung endothelial cells from pyroptosis in sepsis
Abstract
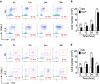
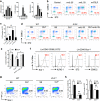
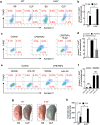
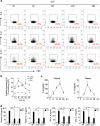
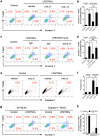
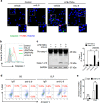
Group 2 innate lymphoid cells (ILC2) are one of three subgroups of innate lymphoid cells (ILC1, ILC2, and ILC3), and the major ILC population detected in the lungs. The function of ILC2 in the regulation of lung inflammation remains unclear. In the current study, we explored an important role of ILC2 in protecting lung endothelial cell (EC) from pyroptosis in sepsis-induced acute lung inflammation and the underlying mechanism. Using a cecal ligation and puncture (CLP) mouse sepsis model, we demonstrated that IL-33, which is released in response to sepsis, acting through its receptor ST2 mediates ILC2 expansion in the lungs. We further showed that the increased ILC2 in the lungs secrete IL-9, which in turn prevents lung EC from undergoing pyroptosis, a pro-inflammatory cell death form, by attenuating caspase-1 activation. These findings suggest a previously unidentified innate pathway that negatively regulates lung inflammation following sepsis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Neuronal-Activated ILC2s Promote IL-17A Production in Lung γδ T Cells During Sepsis.Front Immunol. 2021 Apr 30;12:670676. doi: 10.3389/fimmu.2021.670676. eCollection 2021. Front Immunol. 2021. PMID: 33995408 Free PMC article.
-
Group 2 innate lymphoid cells derived IL-9 reduces macrophage apoptosis and attenuates acute lung injury in sepsis.Inflamm Res. 2025 Feb 13;74(1):38. doi: 10.1007/s00011-024-01978-2. Inflamm Res. 2025. PMID: 39945866
-
Interleukin-33 contributes to ILC2 activation and early inflammation-associated lung injury during abdominal sepsis.Immunol Cell Biol. 2018 Oct;96(9):935-947. doi: 10.1111/imcb.12159. Epub 2018 May 17. Immunol Cell Biol. 2018. PMID: 29672927 Free PMC article.
-
The Role of Innate Lymphoid Cells in the Regulation of Immune Homeostasis in Sepsis-Mediated Lung Inflammation.Diagnostics (Basel). 2020 Oct 12;10(10):808. doi: 10.3390/diagnostics10100808. Diagnostics (Basel). 2020. PMID: 33053762 Free PMC article. Review.
-
Isolation and characterization of mouse innate lymphoid cells.Curr Protoc Immunol. 2014 Aug 1;106:3.25.1-3.25.13. doi: 10.1002/0471142735.im0325s106. Curr Protoc Immunol. 2014. PMID: 25081911 Review.
Cited by
-
Commentary: Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation.Front Immunol. 2018 Jul 2;9:1522. doi: 10.3389/fimmu.2018.01522. eCollection 2018. Front Immunol. 2018. PMID: 30013571 Free PMC article. No abstract available.
-
Macrophage ferroportin serves as a therapeutic target against bacteria-induced acute lung injury by promoting barrier restoration.iScience. 2022 Dec 1;25(12):105698. doi: 10.1016/j.isci.2022.105698. eCollection 2022 Dec 22. iScience. 2022. PMID: 36567719 Free PMC article.
-
Identification of immunogenic cell death-related gene classification patterns and immune infiltration characterization in ischemic stroke based on machine learning.Front Cell Neurosci. 2022 Dec 19;16:1094500. doi: 10.3389/fncel.2022.1094500. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36601430 Free PMC article.
-
The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases.Front Immunol. 2021 Jun 9;12:586078. doi: 10.3389/fimmu.2021.586078. eCollection 2021. Front Immunol. 2021. PMID: 34177881 Free PMC article. Review.
-
Neuronal-Activated ILC2s Promote IL-17A Production in Lung γδ T Cells During Sepsis.Front Immunol. 2021 Apr 30;12:670676. doi: 10.3389/fimmu.2021.670676. eCollection 2021. Front Immunol. 2021. PMID: 33995408 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

