Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop
- PMID: 29511193
- PMCID: PMC5840416
- DOI: 10.1038/s41467-018-03231-x
Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop
Abstract
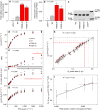
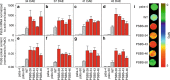
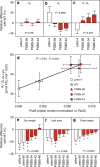
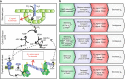
Insufficient water availability for crop production is a mounting barrier to achieving the 70% increase in food production that will be needed by 2050. One solution is to develop crops that require less water per unit mass of production. Water vapor transpires from leaves through stomata, which also facilitate the influx of CO2 during photosynthetic assimilation. Here, we hypothesize that Photosystem II Subunit S (PsbS) expression affects a chloroplast-derived signal for stomatal opening in response to light, which can be used to improve water-use efficiency. Transgenic tobacco plants with a range of PsbS expression, from undetectable to 3.7 times wild-type are generated. Plants with increased PsbS expression show less stomatal opening in response to light, resulting in a 25% reduction in water loss per CO2 assimilated under field conditions. Since the role of PsbS is universal across higher plants, this manipulation should be effective across all crops.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Absence of OsβCA1 causes a CO2 deficit and affects leaf photosynthesis and the stomatal response to CO2 in rice.Plant J. 2017 Apr;90(2):344-357. doi: 10.1111/tpj.13497. Epub 2017 Mar 20. Plant J. 2017. PMID: 28142196
-
Up-regulation of non-photochemical quenching improves water use efficiency and reduces whole-plant water consumption under drought in Nicotiana tabacum.J Exp Bot. 2024 Jul 10;75(13):3959-3972. doi: 10.1093/jxb/erae113. J Exp Bot. 2024. PMID: 38470077 Free PMC article.
-
The photosynthetic response of tobacco plants overexpressing ice plant aquaporin McMIPB to a soil water deficit and high vapor pressure deficit.J Plant Res. 2013 Jul;126(4):517-27. doi: 10.1007/s10265-013-0548-4. Epub 2013 Jan 31. J Plant Res. 2013. PMID: 23371744 Free PMC article.
-
Stomatal Development and Perspectives toward Agricultural Improvement.Cold Spring Harb Perspect Biol. 2019 May 1;11(5):a034660. doi: 10.1101/cshperspect.a034660. Cold Spring Harb Perspect Biol. 2019. PMID: 30988007 Free PMC article. Review.
-
Light-driven modulation of plant response to water deficit. A review.Funct Plant Biol. 2025 Apr;52:FP24295. doi: 10.1071/FP24295. Funct Plant Biol. 2025. PMID: 40261980 Review.
Cited by
-
Blue Light Acclimation Reduces the Photoinhibition of Phalaenopsis aphrodite (Moth Orchid).Int J Mol Sci. 2020 Aug 26;21(17):6167. doi: 10.3390/ijms21176167. Int J Mol Sci. 2020. PMID: 32859101 Free PMC article.
-
Arbuscular Mycorrhizal Symbiosis Enhances Photosynthesis in the Medicinal Herb Salvia fruticosa by Improving Photosystem II Photochemistry.Plants (Basel). 2020 Jul 30;9(8):962. doi: 10.3390/plants9080962. Plants (Basel). 2020. PMID: 32751534 Free PMC article.
-
Investigating the microstructure of plant leaves in 3D with lab-based X-ray computed tomography.Plant Methods. 2018 Nov 12;14:99. doi: 10.1186/s13007-018-0367-7. eCollection 2018. Plant Methods. 2018. PMID: 30455724 Free PMC article.
-
Chloroplastic photoprotective strategies differ between bundle sheath and mesophyll cells in maize (Zea mays L.) Under drought.Front Plant Sci. 2022 Jul 14;13:885781. doi: 10.3389/fpls.2022.885781. eCollection 2022. Front Plant Sci. 2022. PMID: 35909748 Free PMC article.
-
Optical topometry and machine learning to rapidly phenotype stomatal patterning traits for maize QTL mapping.Plant Physiol. 2021 Nov 3;187(3):1462-1480. doi: 10.1093/plphys/kiab299. Plant Physiol. 2021. PMID: 34618057 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

