lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2
- PMID: 29511451
- PMCID: PMC5835822
lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2
Abstract
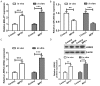
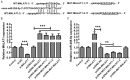
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), as a long chain non-coding RNA (lncRNA), has been reported to be upregulated in Parkinson's disease (PD). However, the mechanisms underlying this process remain unknown. Hence, to investigate the role of MALAT1 in PD, N-methyl-4-phenylpyridinium (MPP+) was used to induce PD in vitro in the MN9D dopaminergic neuronal cell line and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was used to induce PD in vivo in C57BL/6 mice. Quantitative Real-Time PCR (qRT-PCR) and western blot assay showed that the expression levels of MALAT1 and leucine-rich repeat kinase (LRRK2) were increased, and that of miR-205-5p was decreased in the midbrains of mice in which PD was induced by MPTP. MALAT1 suppressed the expression of miR-205-5p in MN9D cells. The results of luciferase reporter assay indicated that LRRK2 was a direct target of miR-205-5p. Transfection with the miR-205-5p mimics decreased, whereas transfection with miR-205-5p inhibitor increased the expression levels of LRRK2 mRNA and protein. The cell counting kit-8 (CCK-8) and flow cytometry assays showed that overexpression of LRRK2 reduced the viability and promoted apoptosis in MN9D cells treated with MPP+. MALAT1 knockdown exerted a protective effect on the viability and apoptosis of MN9D cells treated with MPP+, which was abrogated by LRRK2 overexpression and miR-205-5p inhibition. Our study demonstrates that the MALAT1/miR-205-5p axis regulates MPP+-induced apoptosis in MN9D cells by targeting LRRK2, thereby improving our understanding of the molecular pathogenesis of PD.
Keywords: LRRK2; MALAT1; Parkinson’s disease; apoptosis; long non-coding RNA; miR-205-5p.
Conflict of interest statement
None.
Figures



References
-
- Ma CL, Su L, Xie JJ, Long JX, Wu P, Gu L. The prevalence and incidence of Parkinson’s disease in China: a systematic review and meta-analysis. J Neural Transm (Vienna) 2014;121:123–134. - PubMed
-
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. - PubMed
-
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
