Enantioselective and diastereoselective azo-coupling/iminium-cyclizations: a unified strategy for the total syntheses of (-)-psychotriasine and (+)-pestalazine B
- PMID: 29511522
- PMCID: PMC5659145
- DOI: 10.1039/c5sc00338e
Enantioselective and diastereoselective azo-coupling/iminium-cyclizations: a unified strategy for the total syntheses of (-)-psychotriasine and (+)-pestalazine B
Abstract
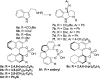
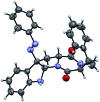
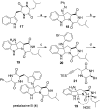
We report a unified strategy for the total syntheses of (-)-psychotriasine and (+)-pestalazine B based on the advanced intermediates of 3α-amino-hexahydropyrrolo[2,3-b]indole. To construct these structural motifs, a cascade reaction involving a BINOL-derived phosphoric anion-paired catalyst for enantioselective or diastereoselective azo-coupling/iminium-cyclizations was developed. The remaining key steps of the synthesis involve a sterically hindered amination via hypervalent iodine reagents and the Larock annulation. These transformations enable a general approach to the syntheses of indole alkaloids containing a 3α-amino-hexahydropyrrolo[2,3-b]indole motif and could be further applied to build a natural product-based library.
Figures




Similar articles
-
Unified Approach to the Spiro(pyrrolidinyl-oxindole) and Hexahydropyrrolo[2,3-b]indole Alkaloids: Total Syntheses of Pseudophrynamines 270 and 272A.Org Lett. 2015 Dec 4;17(23):5922-5. doi: 10.1021/acs.orglett.5b03082. Epub 2015 Nov 24. Org Lett. 2015. PMID: 26600374
-
Intramolecular dearomative oxidative coupling of indoles: a unified strategy for the total synthesis of indoline alkaloids.Acc Chem Res. 2015 Mar 17;48(3):702-11. doi: 10.1021/ar5004303. Epub 2015 Feb 10. Acc Chem Res. 2015. PMID: 25667972
-
Metric-Based Analysis of Convergence in Complex Molecule Synthesis.Acc Chem Res. 2021 Feb 16;54(4):903-916. doi: 10.1021/acs.accounts.0c00817. Epub 2021 Feb 1. Acc Chem Res. 2021. PMID: 33523640
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
-
Evolution of a Strategy for the Unified, Asymmetric Total Syntheses of DMOA-Derived Spiromeroterpenoids.J Org Chem. 2024 Sep 6;89(17):11891-11908. doi: 10.1021/acs.joc.4c01116. Epub 2024 Aug 12. J Org Chem. 2024. PMID: 39133739 Free PMC article. Review.
Cited by
-
Total synthesis of complex 2,5-diketopiperazine alkaloids.Alkaloids Chem Biol. 2023;90:159-206. doi: 10.1016/bs.alkal.2023.06.002. Epub 2023 Aug 7. Alkaloids Chem Biol. 2023. PMID: 37716796 Free PMC article. Review.
-
Enantioselective Synthesis of Pyrroloindolines via Noncovalent Stabilization of Indole Radical Cations and Applications to the Synthesis of Alkaloid Natural Products.J Am Chem Soc. 2018 Mar 7;140(9):3394-3402. doi: 10.1021/jacs.7b13616. Epub 2018 Feb 21. J Am Chem Soc. 2018. PMID: 29432006 Free PMC article.
-
Synthesis of indole derivatives as prevalent moieties present in selected alkaloids.RSC Adv. 2021 Oct 15;11(53):33540-33612. doi: 10.1039/d1ra05972f. eCollection 2021 Oct 8. RSC Adv. 2021. PMID: 35497516 Free PMC article. Review.
-
Cu(i) catalysis for selective condensation/bicycloaromatization of two different arylalkynes: direct and general construction of functionalized C-N axial biaryl compounds.Chem Sci. 2021 Dec 13;13(1):263-273. doi: 10.1039/d1sc03865f. eCollection 2021 Dec 22. Chem Sci. 2021. PMID: 35059176 Free PMC article.
-
Mechanistic duality of indolyl 1,3-heteroatom transposition.Chem Sci. 2023 Jun 16;14(28):7688-7698. doi: 10.1039/d3sc00716b. eCollection 2023 Jul 19. Chem Sci. 2023. PMID: 37476715 Free PMC article.
References
-
- Ruiz-Sanchis P., Savina S. A., Albericio F., Alvarez M. Chem.–Eur. J. 2011;17:1388. - PubMed
-
- Takayama H., Mori I., Kitajima M., Aimi N., Lajis N. H. Org. Lett. 2004;6:2945. - PubMed
-
-
Leading studies on total synthesis of psychotrimine:
- Matsuda Y., Kitajima M., Takayama H. Org. Lett. 2008;10:125. - PubMed
- Newhouse T., Baran P. S. J. Am. Chem. Soc. 2008;130:10886. - PubMed
- Takahashi N., Ito T., Matsuda Y., Kogure N., Kitajima M., Takayama H. Chem. Commun. 2010;46:2501. - PubMed
- Newhouse T., Lewis C. A., Eastman K. J., Baran P. S. J. Am. Chem. Soc. 2010;132:7119. - PMC - PubMed
- Araki T., Ozawa T., Yokoe H., Kanematsu M., Yoshida M., Shishido K. Org. Lett. 2013;15:200. - PubMed
- Zhang H., Kang H., Hong L., Dong W., Li G., Zheng X., Wang R. Org. Lett. 2014;16:2394. - PubMed
-
-
- Waksman S. A., Bugie E. J. Bacteriol. 1944;48:527. - PMC - PubMed
- Mcinnes A. G., Taylor A., Walter J. A. J. Am. Chem. Soc. 1976;98:6741. - PubMed
- Kung A. L., Zabludoff S. D., France D. S., Freedman S. J., Tanner E. A., Vieira A., Cornell-Kennon S., Lee J., Wang B. Q., Wang J. M., Memmert K., Naegeli H. U., Petersen F., Eck M. J., Bair K. W., Wood A. W., Livingston D. M. Cancer Cell. 2004;6:33. - PubMed
- Cook K. M., Hilton S. T., Mecinovic J., Motherwell W. B., Figg W. D., Schofield C. J. J. Biol. Chem. 2009;284:26831. - PMC - PubMed
- Welch T. R., Williams R. M. Tetrahedron. 2013;69:770. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

