A prochelator peptide designed to use heterometallic cooperativity to enhance metal ion affinity
- PMID: 29511523
- PMCID: PMC5659173
- DOI: 10.1039/c5sc00602c
A prochelator peptide designed to use heterometallic cooperativity to enhance metal ion affinity
Abstract
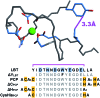
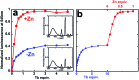
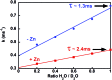
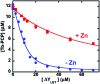
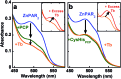
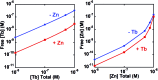
A peptide has been designed so that its chelating affinity for one type of metal ion regulates its affinity for a second, different type of metal ion. The prochelator peptide (PCP), which is a fusion of motifs evocative of calcium loops and zinc fingers, forms a 1 : 2 Zn : peptide complex at pH 7.4 that increases its affinity for Zn2+ ∼3-fold in the presence of Tb3+ (log β2 from 13.8 to 14.3), while the 1 : 1 luminescent complex with Tb3+ is brighter, longer lived, and 20-fold tighter in the presence of Zn2+ (log K from 6.2 to 7.5). This unique example of cooperative, heterometallic allostery in a biologically compatible construct suggests the possibility of designing conditionally active metal-binding agents that could respond to dynamic changes in cellular metal status.
Figures

Similar articles
-
Stimulus-Responsive Prochelators for Manipulating Cellular Metals.Acc Chem Res. 2016 Nov 15;49(11):2468-2477. doi: 10.1021/acs.accounts.6b00380. Epub 2016 Oct 17. Acc Chem Res. 2016. PMID: 27749047 Free PMC article.
-
Cooperative metal binding and helical folding in model peptides of treble-clef zinc fingers.Chemistry. 2009;15(19):4798-810. doi: 10.1002/chem.200900147. Chemistry. 2009. PMID: 19388025
-
Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42.J Neurochem. 2000 Sep;75(3):1219-33. doi: 10.1046/j.1471-4159.2000.0751219.x. J Neurochem. 2000. PMID: 10936205
-
Evaluation of the metal binding properties of a histidine-rich fusogenic peptide by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry.J Mass Spectrom. 2003 Nov;38(11):1150-9. doi: 10.1002/jms.532. J Mass Spectrom. 2003. PMID: 14648822
-
Femtomolar Zn(II) affinity in a peptide-based ligand designed to model thiolate-rich metalloprotein active sites.Inorg Chem. 2006 Dec 11;45(25):9941-58. doi: 10.1021/ic052190q. Inorg Chem. 2006. PMID: 17140191
Cited by
-
Stimulus-Responsive Prochelators for Manipulating Cellular Metals.Acc Chem Res. 2016 Nov 15;49(11):2468-2477. doi: 10.1021/acs.accounts.6b00380. Epub 2016 Oct 17. Acc Chem Res. 2016. PMID: 27749047 Free PMC article.
References
-
- Folk D. S., Kielar F. and Franz K. J., in Comprehensive Inorganic Chemistry II, ed. J. Reedijk and K. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 207–240.
LinkOut - more resources
Full Text Sources
Other Literature Sources

