Regucalcin confers resistance to amyloid-β toxicity in neuronally differentiated PC12 cells
- PMID: 29511612
- PMCID: PMC5832982
- DOI: 10.1002/2211-5463.12374
Regucalcin confers resistance to amyloid-β toxicity in neuronally differentiated PC12 cells
Abstract
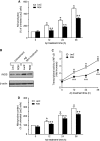
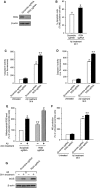
Amyloid-β (Aβ), a primary component of amyloid plaques, has been widely associated with the pathogenesis of Alzheimer's disease. The Ca2+-binding protein regucalcin (RGN) plays multiple roles in maintaining cell functions by regulating intracellular calcium homeostasis, various signaling pathways, and gene expression systems. Here, we investigated the functional role of RGN against Aβ-induced cytotoxicity in neuronally differentiated PC12 cells. Overexpression of RGN reduced Aβ-induced apoptosis by reducing mitochondrial dysfunction and caspase activation. It also attenuated Aβ-induced reactive oxygen species production and oxidative damage and decreased Aβ-induced nitric oxide (NO) overproduction, upregulation of inducible NO synthase by nuclear factor-κB, and nitrosative damage. Interestingly, the genetic disruption of RGN increased the susceptibility of neuronally differentiated PC12 cells to Aβ toxicity. Thus, RGN possesses antioxidant activity against Aβ-induced oxidative and nitrosative stress and may play protective roles against Aβ-induced neurotoxicity in Alzheimer's disease.
Keywords: Alzheimer's disease; amyloid‐β; apoptosis; mitochondrial dysfunction; reactive nitrogen species; reactive oxygen species; regucalcin.
Figures


Similar articles
-
Protective effect of cyclophilin A against Alzheimer's amyloid beta-peptide (25-35)-induced oxidative stress in PC12 cells.Chin Med J (Engl). 2009 Mar 20;122(6):716-24. Chin Med J (Engl). 2009. PMID: 19323941
-
Ergothioneine rescues PC12 cells from beta-amyloid-induced apoptotic death.Free Radic Biol Med. 2004 Feb 1;36(3):288-99. doi: 10.1016/j.freeradbiomed.2003.11.005. Free Radic Biol Med. 2004. PMID: 15036348
-
Neuroprotective effect of epigallocatechin-3-gallate against beta-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity.Arch Pharm Res. 2009 Jun;32(6):869-81. doi: 10.1007/s12272-009-1609-z. Epub 2009 Jun 26. Arch Pharm Res. 2009. PMID: 19557365
-
The Emerging Role of Regucalcin as a Tumor Suppressor: Facts and Views.Curr Mol Med. 2016;16(7):607-619. doi: 10.2174/1566524016666160714124550. Curr Mol Med. 2016. PMID: 27411833 Review.
-
Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders.Neurol Res. 2017 Jan;39(1):73-82. doi: 10.1080/01616412.2016.1251711. Epub 2016 Nov 3. Neurol Res. 2017. PMID: 27809706 Review.
Cited by
-
Regucalcin ameliorates doxorubicin-induced cytotoxicity in Cos-7 kidney cells and translocates from the nucleus to the mitochondria.Biosci Rep. 2022 Jan 28;42(1):BSR20211464. doi: 10.1042/BSR20211464. Biosci Rep. 2022. PMID: 34904631 Free PMC article.
-
Large-scale deep proteomic analysis in Alzheimer's disease brain regions across race and ethnicity.Alzheimers Dement. 2024 Dec;20(12):8878-8897. doi: 10.1002/alz.14360. Epub 2024 Nov 13. Alzheimers Dement. 2024. PMID: 39535480 Free PMC article.
-
Chemical inducer of regucalcin attenuates lipopolysaccharide-induced inflammatory responses in pancreatic MIN6 β-cells and RAW264.7 macrophages.FEBS Open Bio. 2022 Jan;12(1):175-191. doi: 10.1002/2211-5463.13321. Epub 2021 Nov 9. FEBS Open Bio. 2022. PMID: 34709731 Free PMC article.
-
Integration of a neuronal RNAseq dataset with the draft Gryllus bimaculatus transcriptome refines gene predictions and highlights potential systematic response to injury.bioRxiv [Preprint]. 2025 Jul 18:2025.07.13.663756. doi: 10.1101/2025.07.13.663756. bioRxiv. 2025. PMID: 40791512 Free PMC article. Preprint.
-
Regucalcin enhances adipocyte differentiation and attenuates inflammation in 3T3-L1 cells.FEBS Open Bio. 2020 Oct;10(10):1967-1984. doi: 10.1002/2211-5463.12947. Epub 2020 Aug 30. FEBS Open Bio. 2020. PMID: 32783343 Free PMC article.
References
-
- Yamaguchi M and Yamamoto T (1978) Purification of calcium binding substance from soluble fraction of normal rat liver. Chem Pharm Bull 26, 1915–1918. - PubMed
-
- Shimokawa N and Yamaguchi M (1993) Molecular cloning and sequencing of the cDNA coding for a calcium‐binding protein regucalcin from rat liver. FEBS Lett 327, 251–255. - PubMed
-
- Fujita T, Uchida K and Maruyama N (1992) Purification of senescence marker protein‐30 (SMP30) and its androgen‐independent decrease with age in the rat liver. Biochim Biophys Acta 1116, 122–128. - PubMed
-
- Fujita T, Mandel JL, Shirasawa T, Hino O, Shirai T and Maruyama N (1995) Isolation of cDNA clone encoding human homologue of senescence marker protein‐30 (SMP30) and its location on the X chromosome. Biochim Biophys Acta 1263, 249–252. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

