The combined effect of Pdx1 overexpression and Shh manipulation on the function of insulin-producing cells derived from adipose-tissue stem cells
- PMID: 29511614
- PMCID: PMC5832980
- DOI: 10.1002/2211-5463.12378
The combined effect of Pdx1 overexpression and Shh manipulation on the function of insulin-producing cells derived from adipose-tissue stem cells
Abstract
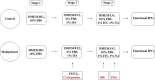
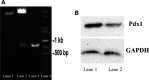
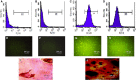
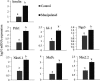
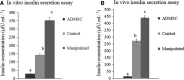
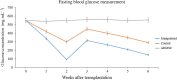
Pancreatic and duodenal homeobox 1 (Pdx1) and Sonic hedgehog (Shh) are the key regulators of beta-cell function. In vitro experiments have shown that there is significant cooperation between Pdx1 and Shh with regard to the production and maintenance of insulin-producing cells (IPCs). In this study, the combined effect of Pdx1 overexpression and Shh manipulation on the function of adipose tissue-derived IPCs was determined. A eukaryotic expression vector (Pdx1- pCDNA3.1(+)) was constructed and transfected into a Chinese hamster ovary (CHO) cell line. Adipose tissue-derived mesenchymal stem cells (ADMSCs) obtained from rats were assigned to two groups [control (C) and manipulated (M)] and differentiated into IPCs. Manipulated cells were treated with a mixture of FGF-β and cyclopamine and recombinant Shh protein at days 3 and 11, respectively, and transfected with Pdx1- pCDNA3.1(+) at day 10. The expression of multiple genes related to function of beta cells was analyzed using real-time PCR. The functionality of IPCs in vitro was analyzed through dithizone (DTZ) staining and ELISA. IPCs were injected into the tail vein of diabetic rats, and blood glucose and insulin concentrations were measured. CHO cells transfected with Pdx1- pCDNA3.1(+) showed a significantly higher expression of Pdx1 compared with nontransfected cells. Manipulated IPCs exhibited a significantly higher expression of MafA, Nkx2.2, Nkx6.1, Ngn3, insulin, and Isl1 and a higher insulin secretion in response to glucose challenge in relation to control cells. Rats that received manipulated IPCs exhibited a higher ability to normalize blood glucose and insulin secretion when compared to controls. Our protocol might be used for more efficient cell therapy of patients with diabetes in the future.
Keywords: Pdx1; adipose tissue‐derived mesenchymal stem cells; insulin‐producing cells; sonic hedgehog pathway.
Figures

References
-
- Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S and Gallicano GI (2012) Stem cell‐based treatments for type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med 29, 14–23. - PubMed
-
- Bottino R, Lemarchand P, Trucco M and Giannoukakis N (2003) Gene‐ and cell‐based therapeutics for type I diabetes mellitus. Gene Ther 10, 875–889. - PubMed
-
- Iype T, Francis J, Garmey JC, Schisler JC, Nesher R, Weir GC, Becker TC, Newgard CB, Griffen SC and Mirmira RG (2005) Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx‐1. J Biol Chem 280, 16798–16807. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

