Effect of a neural relay on liver regeneration in mice: activation of serotonin release from the gastrointestinal tract
- PMID: 29511622
- PMCID: PMC5832978
- DOI: 10.1002/2211-5463.12382
Effect of a neural relay on liver regeneration in mice: activation of serotonin release from the gastrointestinal tract
Retraction in
-
RETRACTION: Effect of a Neural Relay on Liver Regeneration in Mice: Activation of Serotonin Release from the Gastrointestinal Tract.FEBS Open Bio. 2025 May;15(5):889. doi: 10.1002/2211-5463.13961. Epub 2024 Dec 25. FEBS Open Bio. 2025. PMID: 39722559 Free PMC article.
Abstract
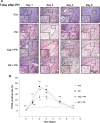
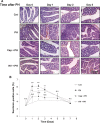
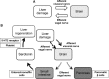
The development of therapeutic options to promote hepatic regeneration following severe liver injury is essential. While humoral factors have been reported as mechanisms of liver regeneration, the contributions of interorgan communication to liver regeneration have not been reported. In this study, we examined the effect of a neural relay on liver regeneration via activation of serotonin release from the gastrointestinal (GI) tract. Our results demonstrated that the afferent visceral nerve from the liver activates the efferent vagus nerve from the brain, leading to activation of serotonin release from the GI tract and contributing to liver regeneration. While it is difficult to apply these results directly to human health, we believe that this study may represent a step toward developing essential therapeutics to promote liver regeneration.
Keywords: gastrointestinal tract; hormone; liver regeneration; neural relay; serotonin.
Figures




Similar articles
-
Autonomic nervous system network and liver regeneration.World J Gastroenterol. 2018 Apr 21;24(15):1616-1621. doi: 10.3748/wjg.v24.i15.1616. World J Gastroenterol. 2018. PMID: 29686468 Free PMC article. Review.
-
Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives.Cell Mol Life Sci. 2008 Mar;65(6):940-52. doi: 10.1007/s00018-007-7377-3. Cell Mol Life Sci. 2008. PMID: 18080089 Free PMC article. Review.
-
Oxytocin in growth, reproduction, restoration and health.Compr Psychoneuroendocrinol. 2024 Sep 30;20:100268. doi: 10.1016/j.cpnec.2024.100268. eCollection 2024 Nov. Compr Psychoneuroendocrinol. 2024. PMID: 39435014 Free PMC article. Review.
-
Vagus nerve cholinergic circuitry to the liver and the gastrointestinal tract in the neuroimmune communicatome.Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G651-G658. doi: 10.1152/ajpgi.00195.2018. Epub 2018 Jul 12. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 30001146 Free PMC article. Review.
-
Electroacupuncture Promotes Liver Regeneration by Activating DMV Acetylcholinergic Neurons-Vagus-Macrophage Axis in 70% Partial Hepatectomy of Mice.Adv Sci (Weinh). 2024 Aug;11(32):e2402856. doi: 10.1002/advs.202402856. Epub 2024 Jun 25. Adv Sci (Weinh). 2024. PMID: 38923873 Free PMC article.
Cited by
-
Involvement of the liver-gut peripheral neural axis in nonalcoholic fatty liver disease pathologies via hepatic HTR2A.Dis Model Mech. 2022 Jul 1;15(7):dmm049612. doi: 10.1242/dmm.049612. Epub 2022 Jul 26. Dis Model Mech. 2022. PMID: 35765850 Free PMC article.
-
Cell Sources and Influencing Factors of Liver Regeneration: A Review.Med Sci Monit. 2020 Dec 14;26:e929129. doi: 10.12659/MSM.929129. Med Sci Monit. 2020. PMID: 33311428 Free PMC article. Review.
-
A Novel Perspective on Neuronal Control of Anatomical Patterning, Remodeling, and Maintenance.Int J Mol Sci. 2023 Aug 29;24(17):13358. doi: 10.3390/ijms241713358. Int J Mol Sci. 2023. PMID: 37686164 Free PMC article. Review.
-
Serotonin as a Mitogen in the Gastrointestinal Tract: Revisiting a Familiar Molecule in a New Role.Cell Mol Gastroenterol Hepatol. 2021;12(3):1093-1104. doi: 10.1016/j.jcmgh.2021.05.008. Epub 2021 May 19. Cell Mol Gastroenterol Hepatol. 2021. PMID: 34022423 Free PMC article. Review.
-
β-adrenergic receptor agonist promotes ductular expansion during 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced chronic liver injury.Sci Rep. 2023 May 1;13(1):7084. doi: 10.1038/s41598-023-33882-w. Sci Rep. 2023. PMID: 37127664 Free PMC article.
References
-
- Iwai M, Cui TX, Kitamura H, Saito M and Shimazu T (2001) Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine 13, 60–64. - PubMed
-
- Yang L, Magness ST, Bataller R, Rippe RA and Brenner DA (2005) NF‐kappaB activation in Kupffer cells after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 289, G530–G538. - PubMed
-
- Nakamura K, Nonaka H, Saito H, Tanaka M and Miyajima A (2004) Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology 39, 635–644. - PubMed
-
- Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B et al (2007) Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest 87, 1018–1028. - PubMed
-
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K and Shimizu S (1989) Molecular cloning and expression of human hepatocyte growth factor. Nature 342, 440–443. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

