Prostaglandin E1 Preconditioning Attenuates Liver Ischemia Reperfusion Injury in a Rat Model of Extrahepatic Cholestasis
- PMID: 29511679
- PMCID: PMC5817361
- DOI: 10.1155/2018/3812424
Prostaglandin E1 Preconditioning Attenuates Liver Ischemia Reperfusion Injury in a Rat Model of Extrahepatic Cholestasis
Abstract
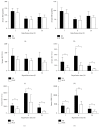
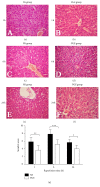
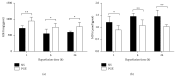
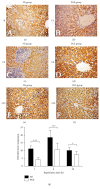
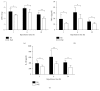
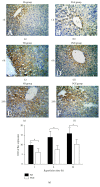
The aim of this study is to explore the hepatoprotective effect of intraportal prostaglandin E1 (PGE1) on liver ischemia reperfusion (IR) injury using an extrahepatic cholestatic model, observing oxidative stress markers, proinflammatory factors, apoptotic marker proteins, and an adhesion molecule. The extrahepatic cholestatic model was induced by common bile duct ligation. After seven days, rats were subjected to ischemia by Pringle maneuver for 15 min, followed by 1, 6, or 24 h of reperfusion. Prostaglandin E1 (PGE group) or normal saline (NS group) was continuously infused from 15 min before liver ischemia to 1 h after reperfusion. After reperfusion, histopathological evaluation of the liver was performed, as were measurements of bilirubin, biochemical enzymes, oxidative stress markers (GSH and MDA), proinflammatory factors (MPO, TNF-α, and IL-1β), apoptotic marker proteins (Bcl-2 and Bax), and the adhesion molecule (ICAM-1). PGE1 pretreatment attenuated IR injury in extrahepatic cholestatic liver probably by suppressing MDA, MPO, TNF-α, IL-1β, ICAM-1, and Bax levels and improving GSH and Bcl-2 levels. In conclusion, PGE1 protects extrahepatic cholestatic liver from IR injury by improving hepatic microcirculation and reducing oxidative stress damage, intrahepatic neutrophil infiltration, and hepatocyte apoptosis.
Figures

Similar articles
-
Ethyl pyruvate prevents intestinal inflammatory response and oxidative stress in a rat model of extrahepatic cholestasis.J Surg Res. 2010 May 15;160(2):228-35. doi: 10.1016/j.jss.2009.03.027. Epub 2009 Apr 21. J Surg Res. 2010. PMID: 19628226
-
Preconditioning with glutamine protects against ischemia/reperfusion-induced hepatic injury in rats with obstructive jaundice.Pharmacology. 2014;93(3-4):155-65. doi: 10.1159/000360181. Epub 2014 Apr 30. Pharmacology. 2014. PMID: 24801881
-
Protective effect of tea polyphenols on renal ischemia/reperfusion injury via suppressing the activation of TLR4/NF-κB p65 signal pathway.Gene. 2014 May 25;542(1):46-51. doi: 10.1016/j.gene.2014.03.021. Epub 2014 Mar 12. Gene. 2014. PMID: 24630969
-
The effect of intraportal prostaglandin E1 on adhesion molecule expression, inflammatory modulator function, and histology in canine hepatic ischemia/reperfusion injury.J Surg Res. 2007 Mar;138(1):88-99. doi: 10.1016/j.jss.2006.05.009. Epub 2006 Dec 15. J Surg Res. 2007. PMID: 17174338
-
A practical approach to extrahepatic cancer screening before and after liver transplant.Clin Liver Dis (Hoboken). 2023 Jun 5;21(6):169-172. doi: 10.1097/CLD.0000000000000060. eCollection 2023 Jun. Clin Liver Dis (Hoboken). 2023. PMID: 37361253 Free PMC article. Review. No abstract available.
Cited by
-
Maternal hepatic immunology during pregnancy.Front Immunol. 2023 Jun 30;14:1220323. doi: 10.3389/fimmu.2023.1220323. eCollection 2023. Front Immunol. 2023. PMID: 37457700 Free PMC article. Review.
References
-
- Wang L., Geng Z.-M., Song X.-G., et al. Concomitant precise hemihepatectomy to improve the efficacy of surgical treatment for hilar cholangiocarcinoma: analysis of 38 cases. Hepato-Gastroenterology. 2014;61(132):927–932. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

