Evaluation of Cerebral White Matter in Prelingually Deaf Children Using Diffusion Tensor Imaging
- PMID: 29511689
- PMCID: PMC5817214
- DOI: 10.1155/2018/6795397
Evaluation of Cerebral White Matter in Prelingually Deaf Children Using Diffusion Tensor Imaging
Abstract
This study compared white matter development in prelingually deaf and normal-hearing children using a tract-based spatial statistics (TBSS) method. Diffusion tensor imaging (DTI) was performed in 21 prelingually deaf (DEAF group) and 20 normal-hearing (HEAR group) subjects aged from 1.7 to 7.7 years. Using TBSS, we evaluated the regions of significant difference in fractional anisotropy (FA) between the groups. Correlations between FA values and age in each group were also analyzed using voxel-wise correlation analyses on the TBSS skeleton. Lower FA values of the white matter tract of Heschl's gyrus, the inferior frontooccipital fasciculus, the uncinate fasciculus, the superior longitudinal fasciculus, and the forceps major were evident in the DEAF group compared with those in the HEAR group below 4 years of age, while the difference was not significant in older subjects. We also found that age-related development of the white matter tracts may continue until 8 years of age in deaf children. These results imply that development of the cerebral white matter tracts is delayed in prelingually deaf children.
Figures
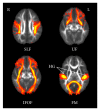
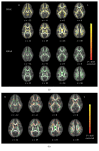
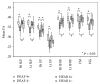
Similar articles
-
Atypical white-matter microstructure in congenitally deaf adults: A region of interest and tractography study using diffusion-tensor imaging.Hear Res. 2017 Jan;343:72-82. doi: 10.1016/j.heares.2016.07.008. Epub 2016 Jul 26. Hear Res. 2017. PMID: 27473505 Free PMC article.
-
Reorganized Brain White Matter in Early- and Late-Onset Deafness With Diffusion Tensor Imaging.Ear Hear. 2021 Jan/Feb;42(1):223-234. doi: 10.1097/AUD.0000000000000917. Ear Hear. 2021. PMID: 32833702
-
Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia.Cortex. 2016 Dec;85:165-181. doi: 10.1016/j.cortex.2016.04.019. Epub 2016 May 4. Cortex. 2016. PMID: 27289586
-
Impaired white matter integrity in infants and young children with autism spectrum disorder: What evidence does diffusion tensor imaging provide?Psychiatry Res Neuroimaging. 2023 Oct;335:111711. doi: 10.1016/j.pscychresns.2023.111711. Epub 2023 Aug 30. Psychiatry Res Neuroimaging. 2023. PMID: 37741094 Review.
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
Cited by
-
The characteristics of brain structural remodeling in patients with unilateral vestibular schwannoma.J Neurooncol. 2023 Mar;162(1):79-91. doi: 10.1007/s11060-023-04247-0. Epub 2023 Feb 20. J Neurooncol. 2023. PMID: 36808599
-
Altered structural connectome of children with auditory processing disorder: a diffusion MRI study.Cereb Cortex. 2023 Jun 8;33(12):7727-7740. doi: 10.1093/cercor/bhad075. Cereb Cortex. 2023. PMID: 36928480 Free PMC article.
-
Assessing Cerebral White Matter Microstructure in Children With Congenital Sensorineural Hearing Loss: A Tract-Based Spatial Statistics Study.Front Neurosci. 2019 Jun 21;13:597. doi: 10.3389/fnins.2019.00597. eCollection 2019. Front Neurosci. 2019. PMID: 31293368 Free PMC article.
-
Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study.Dev Cogn Neurosci. 2019 Aug;38:100654. doi: 10.1016/j.dcn.2019.100654. Epub 2019 May 8. Dev Cogn Neurosci. 2019. PMID: 31129460 Free PMC article.
-
Altered white matter architecture in patients with isolated congenital anosmia.Brain Struct Funct. 2025 May 31;230(5):78. doi: 10.1007/s00429-025-02942-4. Brain Struct Funct. 2025. PMID: 40448824 Free PMC article.
References
-
- Campbell R., Macsweeney M., Woll B. Cochlear implantation (CI) for prelingual deafness: the relevance of studies of brain organization and the role of first language acquisition in considering outcome success. Frontiers in Human Neuroscience. 2014;8, article no. 834 doi: 10.3389/fnhum.2014.00834. - DOI - PMC - PubMed
-
- Nadol J. B. J. R., Shiao J. Y., Burgess B. J., et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110(9):883–891. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

