Dataset on the effects of spermidine on linking number differences between histone H1-free and histone H1-bound circular polynucleosomes
- PMID: 29511714
- PMCID: PMC5832644
- DOI: 10.1016/j.dib.2018.01.091
Dataset on the effects of spermidine on linking number differences between histone H1-free and histone H1-bound circular polynucleosomes
Abstract
The data presented in this article are related to the research article entitled "Quantitative determination of linking number differences between circular polynucleosomes and histone H1-bound circular polynucleosomes" Zhang et al. (in press) [1]. DNA linking number differences between histone H1-free and histone H1-bound circular polynucleosomes at various spermidine concentrations was quantitatively determined by chloroquine-based gel electrophoretic analysis in this work, which provides information on the topological effects of histone H1 and spermidine on the linker DNA between nucleosomes.
Figures
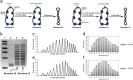

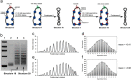

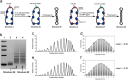

Similar articles
-
Quantitative determination of linking number differences between circular polynucleosomes and histone H1-bound circular polynucleosomes.Bioorg Med Chem Lett. 2018 Feb 1;28(3):537-540. doi: 10.1016/j.bmcl.2017.10.072. Epub 2017 Oct 31. Bioorg Med Chem Lett. 2018. PMID: 29269213
-
Reconstitution of compact polynucleosomes and comparison of the functions of histones H1 and H5.J Biochem. 1984 Oct;96(4):1071-8. doi: 10.1093/oxfordjournals.jbchem.a134924. J Biochem. 1984. PMID: 6520112
-
Characterization of poly(ADP-ribose)--histone H1 complex formation in purified polynucleosomes and chromatin.Eur J Biochem. 1980 Dec;113(1):15-25. doi: 10.1111/j.1432-1033.1980.tb06133.x. Eur J Biochem. 1980. PMID: 7460942
-
Germline-specific H1 variants: the "sexy" linker histones.Chromosoma. 2016 Mar;125(1):1-13. doi: 10.1007/s00412-015-0517-x. Epub 2015 Apr 29. Chromosoma. 2016. PMID: 25921218 Review.
-
Linker histone subtypes and their allelic variants.Cell Biol Int. 2012 Nov 1;36(11):981-96. doi: 10.1042/CBI20120133. Cell Biol Int. 2012. PMID: 23075301 Review.
References
-
- Zhang H., Li T. Quantitative determination of linking number differences between circular polynucleosomes and histone H1-bound circular polynucleosomes. Bioorg. Med. Chem. Lett. 2018 (in press) 28, 2018, 537-540. - PubMed
-
- Zhang H., Li T. Presence of negative supercoiling in aggregates of histone H1-plasmidic polynucleosome complexes. Bioorg. Med. Chem. Lett. 2017;27:168–170. - PubMed
-
- Zhang H., Li T. Effects of spermidine and ATP on stabilities of chromatosomes and histone H1-depleted chromatosomes. Bioorg. Med. Chem. Lett. 2017;27:1149–1153. - PubMed
-
- Bates Andrew D., Maxwell A. The role of ATP in the reactions of type II DNA topoisomerases. Biochem. Soc. Trans. 2010;38:438–442. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

