Patient-reported symptoms and signs of moderate-to-severe psoriasis treated with guselkumab or adalimumab: results from the randomized VOYAGE 1 trial
- PMID: 29512196
- PMCID: PMC6174988
- DOI: 10.1111/jdv.14910
Patient-reported symptoms and signs of moderate-to-severe psoriasis treated with guselkumab or adalimumab: results from the randomized VOYAGE 1 trial
Abstract
Background: How patients experience the symptoms/signs of psoriasis is highly relevant for assessing treatment response.
Objectives: Compare outcomes with guselkumab, placebo and adalimumab utilizing the novel, validated Psoriasis Symptoms and Signs Diary (PSSD).
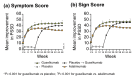
Methods: VOYAGE 1 is an ongoing, phase III, double-blinded, controlled trial of patients with moderate-to-severe psoriasis. Patients were randomized to guselkumab 100 mg every 8 weeks; placebo-to-guselkumab 100 mg every 8 weeks; or adalimumab 40 mg every 2 weeks. The PSSD was self-administered to assess symptoms (i.e. itch, skin tightness, burning, stinging and pain) and signs (i.e. dryness, cracking, scaling, shedding/flaking, redness and bleeding) of psoriasis (0-10 [absent-to-worst-imaginable]) every 24 h. Symptom and sign summary scores were derived (0-100) based on average scores of the individual symptoms and signs. Proportions of patients with clinically meaningful improvements and symptom- and sign-free scores of 0 were evaluated across treatment groups at weeks 16, 24 and 48.
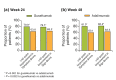
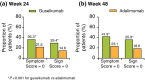
Results: At baseline, 652 of 837 randomized patients had PSSD scores. The proportion of patients achieving clinically meaningful improvements in PSSD summary scores was significantly higher in the guselkumab group compared with the placebo group at week 16 (P < 0.001) and compared with the adalimumab group at weeks 24 (P = 0.002) and 48 (P < 0.001). The proportions of patients achieving PSSD symptom and sign summary scores of 0 (i.e. symptom- and sign-free) were significantly higher for guselkumab vs. placebo at week 16 and vs. adalimumab at weeks 24 and 48 (all P < 0.001).
Conclusions: Based on PSSD scores, greater improvements in symptoms and signs of psoriasis were reported by patients treated with guselkumab compared with placebo at week 16 or adalimumab through 48 weeks.
© 2018 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures
References
-
- Finlay AY, Coles EC. The effect of severe psoriasis on the quality of life of 369 patients. Br J Dermatol 1995; 132: 236–244. - PubMed
-
- Fredriksson T, Pettersson U. Severe psoriasis ‐ oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244. - PubMed
-
- Berth‐Jones J, Grotzinger K, Rainville C et al A study examining inter‐ and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician's Global Assessment and Lattice System Physician's Global Assessment. Br J Dermatol 2006; 155: 707–713. - PubMed
-
- United States Food and Drug Administration . Guidance for Industry: Patient‐reported outcome measures: use in medical product development to support labelling claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf (last accessed on October 24, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

