Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise
- PMID: 29512299
- PMCID: PMC5840389
- DOI: 10.14814/phy2.13628
Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise
Abstract
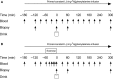
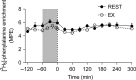
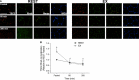
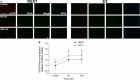
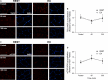
Translocation and colocalization of mechanistic target of rapamycin complex 1 (mTORC1) with regulatory proteins represents a critical step in translation initiation of protein synthesis in vitro. However, mechanistic insight into the control of postprandial skeletal muscle protein synthesis rates at rest and after an acute bout of endurance exercise in humans is lacking. In crossover trials, eight endurance-trained men received primed-continuous infusions of L-[ring-2 H5 ]phenylalanine and consumed a mixed-macronutrient meal (18 g protein, 60 g carbohydrates, 17 g fat) at rest (REST) and after 60 min of treadmill running at 70% VO2peak (EX). Skeletal muscle biopsies were collected to measure changes in phosphorylation and colocalization in the mTORC1-pathway, in addition to rates of myofibrillar (MyoPS) and mitochondrial (MitoPS) protein synthesis. MyoPS increased (P < 0.05) above fasted in REST (~2.1-fold) and EX (~twofold) during the 300 min postprandial period, with no corresponding changes in MitoPS (P > 0.05). TSC2/Rheb colocalization decreased below fasted at 60 and 300 min after feeding in REST and EX (P < 0.01). mTOR colocalization with Rheb increased above fasted at 60 and 300 min after feeding in REST and EX (P < 0.01), which was consistent with an increased phosphorylation 4E-BP1Thr37/46 and rpS6ser240/244 at 60 min. Our data suggest that MyoPS, but not MitoPS, is primarily nutrient responsive in trained young men at rest and after endurance exercise. The postprandial increase in MyoPS is associated with an increase in mTOR/Rheb colocalization and a reciprocal decrease in TSC2/Rheb colocalization and thus likely represent important regulatory events for in vivo skeletal muscle myofibrillar mRNA translation in humans.
Keywords: mTOR; Amino acids; immunofluorescence; mRNA translation; muscle protein synthesis.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures


References
-
- Atherton, P. J. , Etheridge T., Watt P. W., Wilkinson D., Selby A., Rankin D., et al. 2010. Muscle full effect after oral protein: time‐dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 92:1080–1088. - PubMed
-
- Beals, J. W. , Mackenzie R. W., van Vliet S., Skinner S. K., Pagni B. A., and Niemiro G. M., et al. 2017. Protein‐rich food ingestion stimulates mitochondrial protein synthesis in sedentary young adults of different BMIs. J. Clin. Endocrinol. Metab. 102:3415–3424. - PubMed
-
- Beelen, M. , Zorenc A., Pennings B., Senden J. M., Kuipers H., and van Loon L. J.. 2011. Impact of protein coingestion on muscle protein synthesis during continuous endurance type exercise. Am. J. Physiol. Endocrinol. Metab. 300:E945–E954. - PubMed
-
- Bentzinger, C. F. , Romanino K., Cloetta D., Lin S., Mascarenhas J. B., Oliveri F., et al. 2008. Skeletal muscle‐specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 8:411–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

