A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration
- PMID: 29512357
- PMCID: PMC5989759
- DOI: 10.1002/jcsm.12281
A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration
Abstract
Background: Skeletal muscle atrophy induced by either aging (sarcopenia) or mechanical unloading is associated with serious health consequences. Long non-coding RNAs (lncRNAs) are implicated as important regulators in numerous physiological and pathological processes.
Methods: Microarray analysis was performed to identify the differentially expressed lncRNAs in skeletal muscle between adult and aged mice. The most decreased lncRNA in aged skeletal muscle was identified. The C2C12 mouse myoblast cells were used to assess the biological function of the lncRNA in vitro. The target microRNA of lncRNA and the target protein of microRNA were predicted by bioinformatics analysis and validated in vitro. Furthermore, the biology function of the lncRNA in vivo was investigated by local overexpression or knockdown the lncRNA in skeletal muscle. The therapeutic effect of the lncRNA overexpression in age-related or mechanical unloading-induced muscle atrophy was also evaluated.
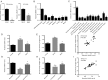
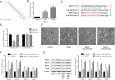
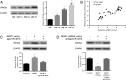
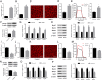
Results: We identified a novel lncRNA (muscle anabolic regulator 1, MAR1) which was highly expressed in mice skeletal muscle and positively correlated with muscle differentiation and growth in vitro and in vivo. We predicted and validated that microRNA-487b (miR-487b) was a direct target of MAR1. We also predicted and validated that Wnt5a, an important regulator during myogenesis, was a target of miR-487b in C2C12 cells. Our findings further demonstrated that enforced MAR1 expression in myoblasts led to derepression of Wnt5a. Moreover, MAR1 promoted skeletal muscle mass/strength and Wnt5a protein level in mice. Enforced MAR1 expression in mice attenuated muscle atrophy induced by either aging or unloading.
Conclusions: The newly identified lncRNA MAR1 acts as a miR-487b sponge to regulate Wnt5a protein, resulting in promoting muscle differentiation and regeneration. MAR1 could be a novel therapeutic target for treating muscle atrophy induced by either aging or mechanical unloading.
Keywords: Long non-coding RNA; Muscle differentiation; Muscle regeneration; Wnt5a; miR-487b.
© 2018 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures



References
-
- Moncaut N, Rigby PW, Carvajal JJ. Dial M(RF) for myogenesis. FEBS J 2013;280:3980–3990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

